Inadequate vascular modeling
leading to infarcts and
insufficiency
– placental villous immaturity and
villous hypoplasia
– possibility of using MCA Dopplers
FGR, fetal growth restriction; MCA, middle cerebral artery; PET, pre-eclampsia; UA, umbilical artery.
Large for dates
At the other extreme are 10% of fetuses born as LFD, which is commonly defined as a birthweight of more than 4,000 g and termed “macrosomic.” Prenatal diagnosis of macrosomic babies remains challenging despite the availability of modern ultrasound machines. It was previously thought that macrosomic babies only pose a problem of maternal or fetal injury at delivery, but evidence is emerging that being LFD is also associated with increased morbidity and mortality, and has implications for future health risks. Although risk factors exist for macrosomia, no combination can predict macrosomia adequately for it to be used clinically. Moreover, many LFD infants have no risk factors.
Etiology of fetal growth disorders
The determination of fetal size is multifactorial and can be divided into maternal, fetal or environmental factors. Failure of one or combination of any of these factors results in abnormal fetal growth (Table 21.2).
APS, antiphospholipid syndrome; CMV, cytomegalovirus; IBD, inflammatory bowel disease; SLE, systemic lupus erythematosus.
Maternal factors
The development of a healthy placenta, vital in maintaining adequate fetal growth, involves invasion and remodeling of the maternal spiral arteries by fetal trophoblast cells. Ideally, this results in a low-resistance circulation, promoting increased blood supply to the placental bed. Any chronic maternal vascular conditions that contribute to abnormal placentation or reduced blood supply to the placental bed can cause FGR. The most common of these are hypertensive disorders, including pre-eclampsia. However, antiphospholipid syndrome appears to carry the highest risk of FGR[2]. Diabetes is more commonly associated with fetal overgrowth, but there is risk of FGR if it is complicated by microvascular disease such as nephropathy. Some examples of other chronic diseases that are associated with FGR are listed in Table 21.2. Placental mosaicism, commonly associated with chromosome 16 and 22 is related to a reduced placental bulk and function, resulting in FGR.
Maternal conditions such as obesity and its associated metabolic changes, including type 2 and gestational diabetes, can result in a baby who is LFD. A macrosomic baby is a sign of poorly controlled diabetes, due to hyperglycemia in the fetus. This stimulates insulin, insulin-like growth factor, growth hormone and other growth factors, which results in stimulation of fetal growth and deposition of fat and glycogen. This theory also holds true for risk of LFD if there is excessive weight gain in pregnancy.
Fetal factors
Fetal chromosomal abnormalities or genetic syndromes are other causes of SGA. The earlier the SGA is detected, the higher the association with chromosomal problems. Congenital anomalies, such as omphalocoele, may increase the risk of chromosomal defects further or may be an independent reason for SGA. Most fetuses of higher order pregnancies are smaller, but may not be FGR.
Although most genetic syndromes are associated with SGA fetus, Beckwith–Wiedermann syndrome is associated with fetal overgrowth. There are usually other features on ultrasound findings, such as macroglossia, organomegaly and abnormal digits, that raise the suspicion of this syndrome in a macrosomic fetus. A fetus that is of advanced gestational age may be large as fetal growth continues in utero.
Environmental factors
Reduced fetal growth is commonly seen in congenital infections, such as cytomegalovirus (CMV), syphilis or rubella. The most common of these is primary CMV infection, which occurs in 1% of the population. There is extensive evidence that both maternal smoking and passive smoking is associated with SGA fetuses. Similarly, alcohol and cocaine use are also linked to being SGA. The National Institute for Health and Clinical Excellence (NICE) in the UK has recommended that women should limit alcohol intake to 1–2 units (1 unit = ½ pint of ordinary strength beer or 25 ml of spirit) once or twice a week.
Screening and diagnosis
Accurate prenatal assessment to identify pregnancies, which may result in abnormal fetal growth, is important to allow for increased fetal monitoring and timely intervention if necessary. Prior to making any diagnosis, it is of upmost importance that the pregnancy has been accurately dated, and the fetus is of the right gestational age. The best method to date a pregnancy is by ultrasound measurements of the crown–rump length in the first trimester.
History
Many risk factors for growth disorders such as advanced maternal age, nulliparity, extremes of body mass index, smoking and poor diet, can be identified at the booking appointment. It is also important to enquire about maternal health status, in particular, chronic diseases and details of previous pregnancies and complications such as SGA or stillbirths.
Customized charts
Growth and birthweights vary with maternal characteristics such as weight at first antenatal-clinic visit, height, parity, and ethnic origin, and other factors, including birthweight of previous babies and fetal sex. However, antenatal population growth charts do not take into account these variables. A growth chart should be computer-generated and “customized” for each individual during pregnancy, taking into consideration the factors stated above[3]. An example is the gestation related optimal weight (GROW) customized charts developed by the Perinatal Institute (www.gestation-net.com) for charting symphyseal fundal height (SFH) and EFW (Figure 21.1).

Example of a completed customized growth chart.
SFH measurements from abdominal palpation involve measuring the distance from the fundus of the uterus to the symphisis pubis, and the distance in centimeters plotted on a customized chart. Unfortunately, SFH measurements have a low sensitivity (27–83%), high intraobserver variability and high false-positive rates for detection of SGA or LFD fetuses, potentially due to inaccuracies from factors such as obesity, lie of fetus, hydramnios, large fibroids and measurements by different practitioners[4]. A single measurement that plots <10th centile, serial measurements that show static growth or cross centiles, or serial measurements that show accelerated growth, should be referred for an ultrasound assessment for EFW and hydramnios, and charted.
On normal population charts, up to 50% of fetuses in the <10th centile category would be normal and constitutionally small. Customized growth charts have reduced the incidence of the normally small fetus, and doubled the detection of FGR from 20–50%. Identification of these FGR pregnancies for appropriate intervention is starting to impact on an improvement in perinatal outcomes, especially reduction in stillbirth rates[5]. The same principle also applies in macrosomia where the baby has exceeded its growth potential if growth plots above the 90th centile on the customized chart. The ultrasound assessment will address the issue of fetal size and polyhydramnios, and should prompt a re-evaluation of the fetus for structural anomalies.
The International Fetal and Newborn Growth Consortium for the 21st Century Project has recently published international growth and size standards for fetuses in a multicenter, population-based setting in eight countries where health and nutritional needs for mothers were met and adequate antenatal care was provided[6]. Named the Fetal Longitudinal Growth Study (FLGS), five ultrasound-based fetal anthropometric measurements were prospectively measured from 14 weeks until birth. The 3rd, 5th, 10th, 50th, 90th, 95th and 97th centile curves were generated for these ultrasound measures, representing the international standards for fetal growth. The researchers found that fetal growth and newborn size at birth were very similar across countries, if the mothers were healthy and well educated. The findings of the study challenge the widely held belief that race and ethnicity are primary factors for weight at birth.
Since publication of the study, there has been widespread debate on the need to customize fetal growth charts based on maternal variables and whether “one size fits all.” More research is currently needed to address this issue.
Maternal serum screening
In view of the placental origin, several biochemical markers, such as alpha-fetoprotein (AFP), human chorionic gonadotropin (hCG), inhibin A, unconjugated estriol (uE3), and pregnancy associated plasma protein-A (PAPP-A), were investigated for screening tests for SGA. Abnormal AFP (>2.5 multiples of the median (MoM) or <0.25 MoM), raised hCG (>3 MoM), and inhibin A (≥2 MoM), low uE3 (<0.5 MoM) and triple test are found to be associated with increased adverse obstetric outcomes, but are of low predictive value for SGA fetuses in systematic reviews[7].
In the first trimester, there is evidence that low PAPP-A (<0.4 MoM) and/or low hCG (<0.5 MoM) are associated with SGA. In view of this, the UK RCOG have included low PAPP-A as a major risk factor for SGA where serial growth and assessment scans are needed throughout pregnancy[2].
There are no serum markers that have been investigated for associations with the fetus that are LFD.
Fetal echogenic bowel
Bright or echogenic fetal bowel at the 20-week anomaly scan is an independent risk factor for SGA and intrauterine death (IUD)[8]. In view of this, serial growth scans and close monitoring is recommended if this is found[2].
Ultrasound assessments
Serial fetal biometry
Ultrasound biometry measurements commonly include head circumference, biparietal diameter, AC and femur length, which will allow an EFW to be calculated from a formula. Of all parameters, fetal AC (<10th centile) has the highest sensitivity for identifying SGA fetuses. However, not all of these fetuses will have low birthweight, and may have unnecessary intervention if AC <10th centile alone were used to identify SGA fetuses. The accuracy of diagnosing SGA increases when both the AC and calculated EFW are <10th centile, and this group of fetuses are at higher risk of perinatal morbidity[9]. There are currently more than 30 published formulas for calculating EFW, taking into account different biometric parameters. Although there are considerable variations in the accuracy, they are generally acceptable for birthweights up to 3500 g. Most formulas overestimate SGA fetuses and underestimate LFD fetuses. In a population at high risk of SGA, the sensitivity for detection ranged from 72–100%, while specificity was 41–88%. In this high-risk population, the Hadlock formula appears to have the optimal sensitivity/specificity trade-off in the detection for SGA[10].
A single set of fetal biometry <10th centile will not distinguish between a constitutionally SGA fetus and FGR. Thus, assessment should include repeated fetal biometry measurements and EFW, charted at each scan to assess for the pattern of fetal growth. There is no consensus on the frequency or optimal time interval between scans. If the aim of two different scans is to measure growth velocity, the interval should be at least 3 weeks to minimize false-positives diagnosis of FGR from scan error.
Liquor volume assessment
The liquor volume can be reported as maximum pool depth (MPD), a single measurement of the deepest vertical pocket of liquor, or amniotic fluid index (AFI), the sum of vertical measurement of liquor in four quadrants of the uterus. A Cochrane review has shown that when AFI was used, there were more diagnoses of oligohydramnios, leading to intervention of induction of labor and cesarean delivery for fetal distress without evidence of improvements in adverse peripartum outcomes, such as admission to neonatal intensive care unit, umbilical artery pH <7.1, and Apgar score <7 at 5 min. Thus, it is recommended that MPD is used as the tool to assess liquor volume[11]. An abnormality that requires further investigation would be MPD of <2 cm or >10 cm.
Uterine artery Doppler
An abnormal uterine artery (UtA) Doppler velocimetry (pulsatility index (PI) >95th centile, mean PI >1.45, and/or notching) correlates with high resistance in the maternal arterial blood supply to the placental bed, secondary to poor trophoblast invasion and remodeling of the maternal spiral arteries (Figures 21.2 and 21.3). A systematic review of 61 studies reported good predictive value of FGR if the UtA Doppler was abnormal with or without notching at 20–24 weeks[12]. In the majority of cases of abnormal UtA Doppler at 20–24 weeks, the PI remains raised when the test is repeated at 26–28 weeks gestation. Even if the PI normalizes, there is still an increased risk of FGR, and thus repeating UtA Dopplers at a later gestation is of limited value. Instead, a surveillance strategy should be in place to identify when FGR occurs.
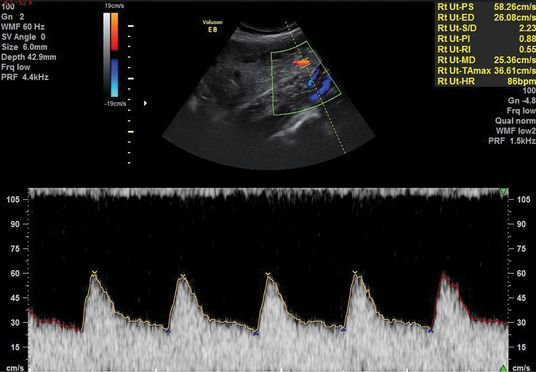
Normal uterine artery Doppler waveform.
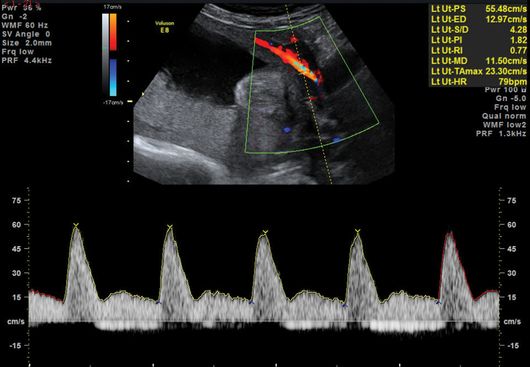
Abnormal uterine artery Doppler waveform with notching.
Fetal Dopplers
Referral for further assessment of detailed Doppler studies depends on the customized growth chart pattern where consecutive measurements show either tapering of growth, static growth or exponential growth. Doppler studies could include umbilical artery (UA), middle cerebral artery (MCA), aortic isthmus, and venous circulation such as ductus venosus (DV), umbilical vein or inferior vena cava to allow for an assessment of the fetal and maternal circulation. Arterial Dopplers assess the placental function and fetal vasoregulation, while venous Dopplers provide information about fetal cardiac function. The commonly used Dopplers in the surveillance of high-risk pregnancies are UA, MCA and DV Dopplers[13].
There should be a low-resistance system with forward flow throughout the cardiac cycle in a normal pregnancy. The UA Doppler evaluates placental function and advancing severity of fetoplacental impairment leads to the progressive increase in the PI and resistence index of the UA Dopplers from a normal waveform to absent end-diastolic flow (EDF), to reversed EDF (Figures 21.4– 21.6). A systematic review of 18 studies on more than 10,000 women showed that use of UA Doppler in monitoring high-risk pregnancies, including SGA, was associated with a reduction in perinatal death from 1.7–1.2% (relative risk 0.71, 95% confidence interval 0.52–0.98), with fewer inductions of labor and cesarean sections[14]. Therefore, as a minimum, UA Doppler should be performed with all serial growth scans.
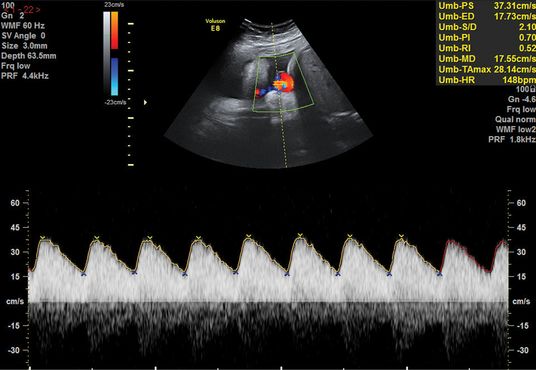
Normal umbilical artery waveform.
Absent end-diastolic flow in the umbilical artery waveform.
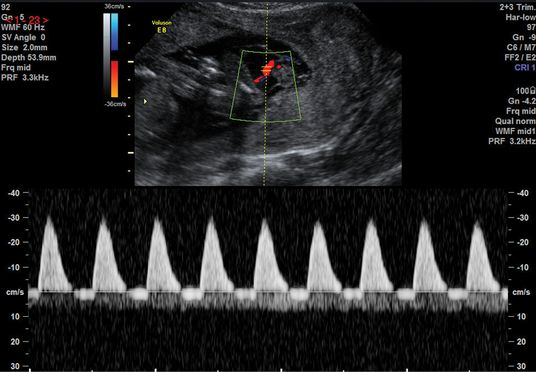
Reversed end-diastolic flow in the umbilical artery waveform.
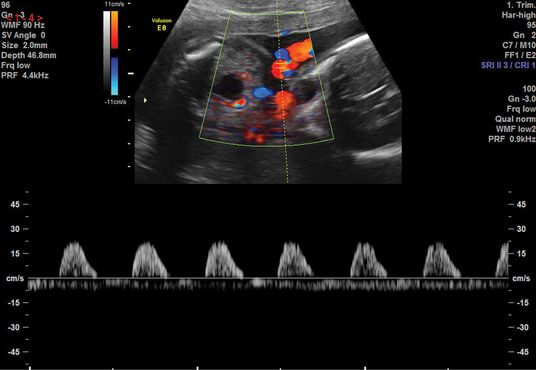
An abnormal flow pattern in the UA Doppler may lead to redistribution of blood flow to allow better oxygenation to vital organs, such as the brain and heart, leading to the “brain-sparing” effect. This is demonstrated by a reduced resistance to blood flow in the MCA (Figures 21.7 and 21.8). Another screening parameter is the cerebroplacental ratio (CPR), which is a measure of two Doppler indices, MCA PI/UA PI, and has been demonstrated to be more sensitive to fetal hypoxia[15]. A decreasing CPR suggests fetal compromise and correlates better with adverse outcome compared to its individual measurements.
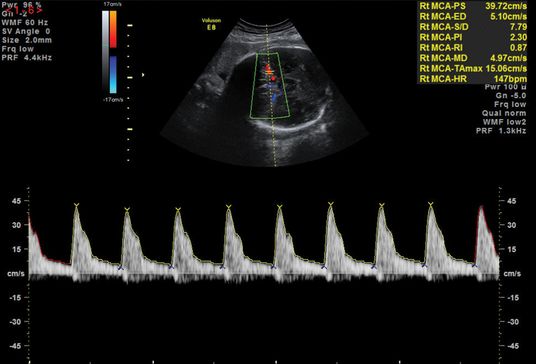
Normal middle cerebral artery waveform.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


