Fetal Cardiology
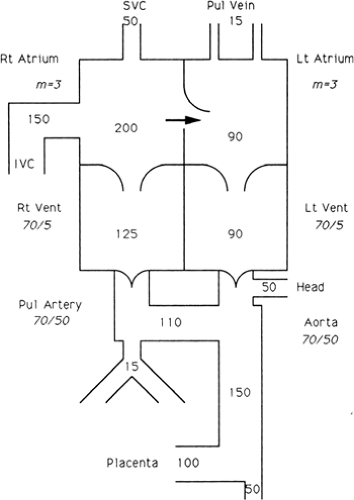
Extensive information about the circulatory physiology of the fetus and newborn has accumulated. The works of Barcroft (37), Dawes (38), Lind and associates (39), and Rudolph (40) should be consulted for details, but the central features are discussed here. The circulation before birth consists of parallel circuits (Fig. 33-1). Blood in the aorta may follow several routes to a capillary bed in the fetus or the placenta, back to the heart, passing through either ventricle, and out again to the aorta. The stream of newly oxygenated blood from the placenta passes through the umbilical vein, the ductus venosus, the inferior vena cava,
and the right atrium. Unlike the circulation after birth, the streams of oxygenated and unoxygenated blood are not completely separated, although the more oxygenated blood from the inferior vena cava is mostly diverted through the foramen ovale into the left atrium. Consequently, blood from the left ventricle entering the ascending aorta and coronary and carotid circulations is somewhat higher in oxygen than that entering the descending aorta from the right ventricle by way of the ductus arteriosus.
and the right atrium. Unlike the circulation after birth, the streams of oxygenated and unoxygenated blood are not completely separated, although the more oxygenated blood from the inferior vena cava is mostly diverted through the foramen ovale into the left atrium. Consequently, blood from the left ventricle entering the ascending aorta and coronary and carotid circulations is somewhat higher in oxygen than that entering the descending aorta from the right ventricle by way of the ductus arteriosus.
The volume pumped by the right ventricle is normally about 55% of the combined output of both ventricles. Because both ventricles pump against the systemic resistance, the level of pressure in the two ventricles is comparable. The resistance to blood flow through the lungs is relatively great; only minimal flow through the lungs occurs in utero, and almost all of the right ventricular output into the pulmonary artery passes through the ductus arteriosus to the descending aorta. The parallel arrangement of the ventricles allows fetal survival despite a wide variety of cardiac lesions. With total obstruction of either ventricle, the other ventricle assumes the entire cardiac output. Reversal of the pulmonary arterial and aortic streams of blood, as occurs in transposition of the great arteries, produces no deleterious effect on the fetus. Additionally, ductal or ascending aortic flow may reverse in the presence of severe semilunar valvar stenosis or atresia. Despite this remarkable ability to adapt, grow, and survive, the fetus is affected by limitations in myocardial contractility. Prolonged, severe pressure or volume loading of the heart or primary myocardial disease may result in congestive heart failure, manifested by hydrops fetalis. The interplay between the metabolic effects of congestion in the fetus and the possible compensatory role of the placenta is not understood. Because lesions that may be expected to cause gross intrauterine difficulty are tolerated surprisingly well, the postulate that the placenta helps compensate for the metabolic abnormalities resulting from congestive heart failure is tenable.
Circulatory Adjustments at Birth
Changes in the Source of Oxygenated Blood and in the Ductus Venosusand Ductus Arteriosus
With the first breath, the resistance to pulmonary blood flow drops sharply. The oxygen content of the left heart and systemic circulation rapidly reaches levels well above that of the fetal circulation. Oxygen saturation in the ascending aorta rises from about 65% in the fetus, to about 93% immediately after birth. The ductus venosus functionally closes, establishing the portal circulation as an independent loop between two capillary beds. With removal of the low-resistance placenta, systemic resistance increases. The relative fall in the pulmonary resistance and rise in the systemic resistance result in a transitory left-to-right shunt through the ductus arteriosus. In half of term babies the ductus is completely constricted by the end of the first day of life, and is normally becoming anatomically obliterated at about 10 days of age. Even among cyanotic newborns who are duct dependent, the ductus may inexorably close, often severing the infant’s only source of pulmonary or systemic blood flow. The mechanisms causing closure of the ductus arteriosus are not completely understood but involve decreased prostaglandins and increased blood oxygen. Prostaglandin levels in the blood decrease at birth as a result of removal of their placental source of production from the circulation and increase in perfusion of the lungs, in which prostaglandins are metabolized.
Foramen Ovale
Functional closure of the foramen ovale occurs soon after birth, largely as a result of increased left atrial volume and pressure secondary to the increased pulmonary venous return, the ductal left-to-right shunt, and the developing differences in diastolic pressure of the two ventricles. Anatomic closure normally is delayed for months or years.
Among infants with cardiac defects, lesions with increased right atrial pressure favor indefinite patency of the foramen ovale (e.g., pulmonary stenosis), but abnormally increased left atrial pressure promotes early anatomic closure (e.g., ventricular septal defect). Before birth, the pulmonary arterioles are relatively muscular and constricted.
Among infants with cardiac defects, lesions with increased right atrial pressure favor indefinite patency of the foramen ovale (e.g., pulmonary stenosis), but abnormally increased left atrial pressure promotes early anatomic closure (e.g., ventricular septal defect). Before birth, the pulmonary arterioles are relatively muscular and constricted.
Pulmonary Vasculature
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree