As this follicle matures, its oestradiol output increases considerably. When a high ‘threshold’ level of oestradiol is attained, the negative feedback is reversed and positive feedback now causes LH and FSH levels to again increase and dramatically so: it is the peak of the former that ultimately leads to rupture of the now ripe follicle when it is around 2 cm diameter. This is ovulation and the egg spills onto the ovarian surface where it can be picked up by the fallopian tube. Following ovulation the follicle becomes a corpus luteum and releases oestrogen and progesterone to maintain a secretory endometrium suitable for embryo implantation. If this does not occur the corpus luteum involutes and hormone levels fall, leading to menstruation around 14 days after ovulation. If embryo implantation does occur, the human chorionic gonadotrophin (hCG) produced by the trophoblast tissue acts on the corpus luteum to maintain oestrogen and progesterone production until the fetoplacental unit takes over at 8–10 weeks’ gestation.
Detection of Ovulation
History:
The vast majority of women with regular cycles are ovulatory. Some experience vaginal spotting or an increase in vaginal discharge or pelvic pain (‘mittelschmertz’) around the time of ovulation.
Examination:
Cervical mucus preovulation is normally acellular, will ‘fern’ (form fern-like patterns) when on a dry slide (Fig. 11.2a) and will form ‘spinnbarkeit’ (elastic-like strings) of up to 15 cm (Fig. 11.2b). The body temperature normally drops some 0.2°C preovulation and then rises 0.5°C in the luteal phase. If the woman is asked to record her temperature every day, the pattern can be seen on a temperature chart (Fig. 11.2c). These examinations are generally not requested or performed.
Fig. 11.2 Evidence of ovulation: (a) cervical mucus showing fern-like pattern; (b) spinnbarkeit formation of mucus between two glass slides; (c) temperature chart.
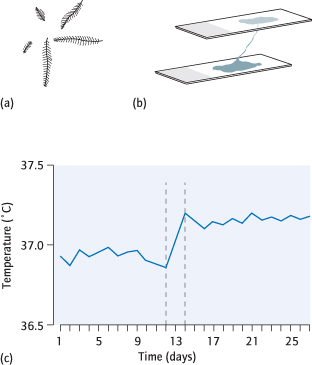
Investigations are more reliable and are used more frequently. The only proof of ovulation is conception but positive investigations are strongly suggestive.
1 Elevated serum progesterone levels in the mid-luteal phase usually indicate that ovulation has occurred. The luteal phase (time from ovulation to subsequent menstruation) is constant at 14 days. Therefore a low progesterone result can only be interpreted as showing lack of ovulation if it was taken around 7 days before the subsequent menstruation, i.e. day 21 of a 28-day cycle or day 28 of a 35-day cycle. For women with irregular cycles repeat progesterone tests may be required until menstruation starts.
2 Ultrasound scans can serially monitor follicular growth and, after ovulation, demonstrate the fall in size and haemorrhagic nature of the corpus luteum. This is time-consuming and generally not performed.
3 Over-the-counter urine predictor kits will indicate if the LH surge has taken place. Ovulation should then follow.
Detection of Ovulation
Mid-luteal phase serum progesterone (the standard test)
Ultrasound follicular tracking (time-consuming)
Temperature charts (not recommended)
Luteinizing hormone (LH) -based urine predictor kits
Causes of Anovulation: Polycystic Ovary Syndrome
Polycystic ovary syndrome (PCOS) is a diagnosis of exclusion. Other causes of irregular or absent periods need to be considered and investigated as appropriate [→ p.16].
Definitions and Epidemiology
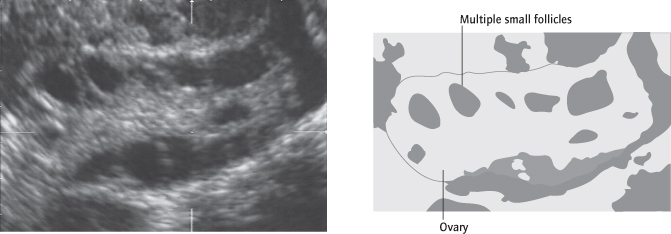
Polycystic ovary (PCO) describes a characteristic transvaginal ultrasound appearance of multiple (12 or more) small (2–8 mm) follicles in an enlarged (>10 mL volume) ovary. PCO is found in about 20% of all women (Fig. 11.3), the majority of whom have regular ovulatory cycles. Women with PCO may develop other features of the full syndrome if they put on weight (see below).
Polycystic Ovary Syndrome:
PCOS affects around 5% of women and causes over 80% of cases of anovulatory infertility. It is diagnosed when at least two out of the following three criteria are met (JCEM 2006: 91; 786): (i) PCO on ultrasound, (ii) irregular periods (>35 days apart) and (iii) hirsutism: clinical (acne or excess body hair) and/or biochemical (raised serum testosterone).
Pathology/Aetiology
Susceptibility to PCO is mainly genetic. Affected women demonstrate disordered LH production and peripheral insulin resistance with compensatory raised insulin levels. The combination of raised levels of LH and insulin acting on the PCO lead to increased ovarian androgen production. Raised insulin levels also increase adrenal androgen production and reduce hepatic production of steroid hormone binding globulin (SHBG) which leads to increased free androgen levels. Increased intraovarian androgens disrupt folliculogenesis leading to excess small ovarian follicles (and the PCO picture) and irregular or absent ovulation. Raised peripheral androgens cause hirsutism (acne and/or excess body hair). Increasing body weight leads to increased insulin and consequently androgen levels. Hence environmental factors (weight) can modify the phenotype of PCOS. Many women have a family history of type II diabetes.
Clinical Features
PCO:
Polycystic ovaries without the syndrome generally cause no symptoms.
PCOS:
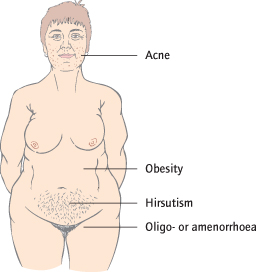
The stereotypical patient with the syndrome is obese, has acne, hirsutism and oligomenorrhoea or amenorrhoea: these may therefore be the presenting symptoms (Fig. 11.4). However, since only two out of the three criteria are necessary presentation can vary. Although many women with severe PCOS have normal body weight, changes in weight over time will alter insulin levels and severity of the syndrome. Miscarriage is more common in PCOS and may be related to the increased levels of LH and/or insulin and also increased body weight.
Diagnosis of Polycystic Ovary Syndrome
Two or more out of:
- Ovaries polycystic morphology on ultrasound
- Irregular periods 5 weeks or more apart
- Hirsutism (clinical and/or biochemical)
Investigations
Alternative causes for the symptoms need to be excluded.
Blood Tests:
Anovulation is investigated with FSH (raised in ovarian failure, low in hypothalamic disease, normal in PCOS), prolactin (to exclude a prolactinoma) and thyroid-stimulating hormone (TSH). Hirsutism is investigated with serum testosterone levels (possibility of androgen-secreting tumour or congenital adrenal hyperplasia if very raised). LH is measured (often raised in PCOS but not diagnostic).
Ultrasound:
(transvaginal scan) is used to look for polycystic ovaries (Fig. 11.3).
Other:
Screening for diabetes and abnormal lipids is also advised, particularly if the woman is obese or has a family history of diabetes, abnormal lipids or cardiovascular disease.
Clinical Features of Polycystic Ovary Syndrome (PCOS)
None
Subfertility
Oligomenorrhoea or amenorrhoea
Hirsutism and/or acne
Obesity
Miscarriage
Investigations for Polycystic Ovary Syndrome (PCOS)
Transvaginal ultrasound scan
Follicle-stimulating hormone (FSH), luteinizing hormone (LH), testosterone, prolactin, thyroid-stimulating hormone (TSH)
Fasting lipids and glucose to screen for complications
Complications of PCOS
Up to 50% of women with PCOS develop type II diabetes in later life; 30% develop gestational diabetes [→ p.183] during pregnancy, a risk reduced by weight reduction. Endometrial cancer is more common in women with many years of amenorrhoea due to unopposed oestrogen action. In spite of a number of risk factors (weight, insulin resistance, diabetes, abnormal lipids) increased mortality rates have not been demonstrated in women with PCOS.
Treatment of Symptoms Other Than Infertility
Advice regarding diet and exercise are given. Normalization of weight should result in reduction in insulin levels and improvement in all PCOS symptoms. If fertility is not required, treatment with the combined oral contraceptive will regulate menstruation and treat hirsutism. At least three to four bleeds per year, whether spontaneous or induced, are necessary to protect the endometrium. The antiandrogens cyproterone acetate (also available combined as a contraceptive pill) or spironolactone are effective treatments for hirsutism but conception must be avoided. The insulin sensitizer metformin reduces insulin levels, and therefore androgens and hirsutism (and also promotes ovulation). Eflornithine is a topical antiandrogen used for facial hirsutism.
Other Causes of Anovulation
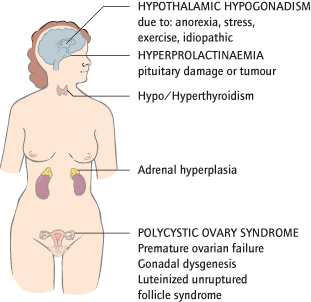
These may originate in the ovary, the pituitary or hypothalamus, or in other parts of the endocrine system (Fig. 11.5). Pregnancy causes amenorrhoea.
Hypothalamic Causes
Hypothalamic Hypogonadism:
A reduction in hypothalamic GnRH release causes amenorrhoea, because reduced stimulation of the pituitary reduces FSH and LH levels, which in turn reduces oestradiol levels. This is usual with anorexia nervosa (Fig. 11.6) and common in women on diets, athletes and those under stress. Restoration of body weight, if appropriate, restores hypothalamic function. Kallmann’s syndrome occurs when GnRH secreting neurones fail to develop; in other patients, the cause is obscure. Exogenous gonadotrophins or a GnRH pump will induce ovulation. Bone protection with the contraceptive pill or hormone replacement therapy (HRT) is required.
Pituitary Causes
Hyperprolactinaemia is excess prolactin secretion, which reduces GnRH release. It is usually caused by benign tumours (adenomas) or hyperplasia of pituitary cells, but is also associated with PCOS, hypothyroidism, and the use of psychotropic drugs. It accounts for 10% of anovulatory women, who commonly have oligomenorrhoea or amenorrhoea, galactorrhoea and, if a pituitary tumour is enlarging, headaches and a bitemporal hemianopia. Prolactin levels are elevated. Computed tomography (CT) imaging is indicated if neurological symptoms occur. Treatment with a dopamine agonist (bromocriptine or cabergoline) usually restores ovulation, because dopamine inhibits prolactin release. Surgery is needed if this fails or neurological symptoms warrant it.
Pituitary damage can reduce FSH and LH release. Production of GnRH is normal. This results from pressure from tumours, or infarction following severe postpartum haemorrhage (Sheehan’s syndrome).
Ovarian Causes of Anovulation (in Addition to PCOS)
Premature Ovarian Failure [→ p.109]:
As the ovary fails, oestradiol and inhibin levels are low, so reduced negative feedback on the pituitary causes FSH and LH levels to rise. Exogenous gonadotrophins are of no use, since there are no ovarian follicles to respond, and donor eggs are required for pregnancy. Bone protection with HRT or the oral contraceptive pill is required.
Gonadal Dysgenesis:
These rare conditions usually present with primary amenorrhoea [→ p.16].
The luteinized unruptured follicle syndrome is present when a follicle develops but the egg is never released. It is unlikely to occur every month so does not cause persistent problems.
Other Causes
Hypo- or hyperthyroidism reduce fertility. Menstrual disturbances are usual.
Androgen-secreting tumours [→ p.17] cause amenorrhoea and virilization.
Common Causes of Anovulation
Polycystic ovary syndrome (PCOS)
Hypothalamic hypogonadism
Hyperprolactinaemia
Thyroid disease
Induction of Ovulation
Lifestyle Changes and Treatment of Associated Disease
Treatment of fertility involves health advice regarding pregnancy, the risks of multiple pregnancy with ovulation induction and the use of folic acid [→ p.146]. Restoration of normal weight is advised: this alone may restore ovulation. Treatment of specific causes, such as a thyroid abnormality or hyperprolactinaemia, usually leads to restoration of ovulation. Smoking should cease.
Treatment of PCOS
Clomifene is the traditional first-line ovulation induction drug in PCOS. It is limited to 6 months’ use and results in ovulation and live birth rates of around 70% and 40%, respectively. Clomifene is an antioestrogen, blocking oestrogen receptors in the hypothalamus and pituitary. As gonadotrophin release is normally inhibited by oestrogen, it increases the release of FSH and LH. Effectively, therefore, it ‘fools’ the pituitary into ‘believing’ there is no oestrogen. As it is only given at the start of the cycle, from days 2 to 6, it can initiate the process of follicular maturation which is thereafter self-perpetuating for that cycle. Clomifene cycles should be monitored by transvaginal ultrasound, at least in the first month, to assess ovarian response (both under and over) and endometrial thickness. If no follicles develop then the dose in subsequent cycles is increased from 50 mg/day to 100 mg and, if necessary, a maximum of 150 mg/day. If three or more follicles develop then cycle cancellation is generally indicated to reduce the risk of multiple pregnancy (overall 10%). As clomifene is an antioestrogen it has negative effects at the endometrium and, on higher doses, may cause a thin endometrium of <7 mm. This might explain the live birth rate (40%), which is lower than expected in view of the good ovulation rates (70%).
If ovulation does not occur despite dose escalation (‘clomifene resistance’) then second-line treatments include:
Metformin is an oral insulin sensitizing drug which aims to restore ovulation. It does not promote multiple ovulation so there is no increase in multiple pregnancies (and no need for scan monitoring). When used alone it has a lower live birth rate compared to clomifene, so clomifene continues to be the first-line treatment of choice (NEJM 2007; 356: 551). Metformin increases the effectiveness of clomifene in clomifene-resistant women. It treats hirsutism so may be a suitable first-line fertility treatment for anovulatory women who want hirsutism treated and to avoid multiple pregnancy. Additional benefits, when metformin is continued during pregnancy, may include a reduction in both early miscarriage and the development of gestational diabetes, which are more common with PCOS.
Laparoscopic ovarian diathermy is as effective as gonadotrophins (Cochrane 2007: CD001122) and with a lower multiple pregnancy rate. Each ovary is monopolar diathermied at a few points for a few seconds. During the same operation tubal patency can be tested using methylene blue insufflation and any co-morbidities such as endometriosis or adhesions treated. If successful then regular ovulations can continue for years. Patients are warned of the risks of surgery, including periovarian adhesion formation and, rarely, ovarian failure.
Gonadotrophins (see below).
Gonadotrophin Induction of Ovulation
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree