Complication
Comments
Oxygenator failure
It is unusual for an oxygenator to fail completely, but they do lose efficiency over time
Pump tubing (raceway) rupture
Only in the roller pump system
Unfavorable cannula position
More common with the venous cannula. Leads to high negative pressures and inadequate flows especially with centrifugal pumps
Circuit thrombus formation
May occur in oxygenator, bridge, and bladder of hemofilter
Air embolism
Can occur if there are cracks in the tube or through stopcocks. There is a high negative pressure in the venous line pre-oxygenator of centrifugal pumps
Pump failure
A rare event. Pumps have a battery backup system which can also be used for transport. There is also a hand crank option
Hemolysis
Formerly a significant problem with the centrifugal pump system. Much less of a problem with the newer pump heads
Table 66.2
Principal patient complications
Complication | Comments |
|---|---|
Seizures and other CNS complications | May be caused by intracerebral hemorrhage or infarction. Commonly manifested by the onset of seizures |
Systemic hypertension | May be a predisposing factor to above. Systemic hypertension is seen frequently when pump flow is initiated |
Renal dysfunction and fluid overload | The development of renal failure or the inability to maintain patient within 10 % of dry weight has been negatively correlated with outcome (Swaniker et al. 2000) |
Culture-proven infection | Can be seen in up to 12 % of patients (Bizzarro et al. 2011) |
Hepatic failure and hyperbilirubinemia | Hepatic failure developing on ECMO is associated with poor outcome (Swaniker et al. 2000) |
Failure to separate from ECMO | Failure of lung recovery and major neurological damage are the most common reasons |
66.5.3 Outcome in ECMO Support for AHRF
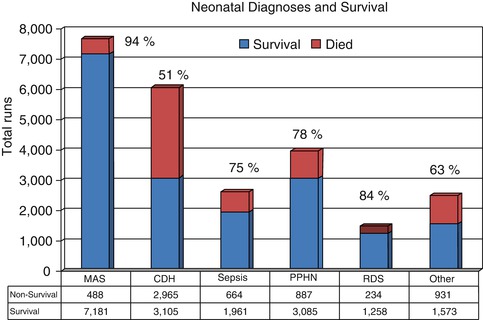
The survival to separation from ECMO in over 24,000 patients placed with AHRF in the newborn period is 85 % in the ELSO Registry with 75 % survival to hospital discharge (ELSO Registry 2010) (Fig. 66.1). Within this large group with various etiologies, the survival is best in meconium aspiration syndrome (>90 %) and worst in congenital diaphragmatic hernia (60 %). The other noticeable trend in the Registry data over the past 5 years has been the decrease in number of neonatal patients where ECMO has been used as a rescue therapy. This is partly due to the increased use of alternate therapies such as iNO and high-frequency ventilation but also due in large part to an alteration in ventilation practices, principally abandoning the use of hyperventilation to reverse ductal shunting. While peak airway pressures of over 40 cmH2O were commonly seen in patients referred for ECMO, there has been a significant reduction in this past decade with the increasing recognition of ventilation-induced lung injury (Roy et al. 2000). A convincing argument can be made that very high numbers of these patients being put on ECMO in the 1990s were being rescued from ventilation-induced lung injury rather than underlying lung discese. However, widespread adoption of these alternate therapies is not without hazard in that when these fail to improve the oxygenation, sicker infants require transfer to an ECMO center, although this has not been reflected in an increase in mortality (Fliman et al. 2006). The argument as to whether ECMO improves the outcome in severe AHRF in newborns has been addressed in three randomized trials. Two of these were criticized because of lack of equipoise in the study sites (Bartlett et al. 1985; O’Rourke et al. 1989). The third was performed in the UK where 55 referral centers randomized 185 patients to either conventional management or transfer to one of five ECMO centers. The primary end point was survival without disability at 1 year. The study was stopped early because of favorable outcome in the ECMO group (UK Collaborative ECMO Trail Group 1996).
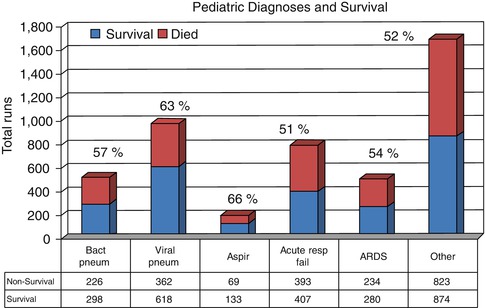
The outcome in the over 4,000 children outside the newborn period reported to ELSO is different with 65 % being successfully separated from ECMO and 56 % surviving to hospital discharge (ELSO Registry 2010) (Fig. 66.2). The average duration of support was 10 days with occasional very prolonged runs of up to 3 months. The fact that the outcome is not as good as the neonatal patients is not surprising given the fact that in the former group the majority of patients have single-system pulmonary disease while older children frequently have multisystem disease.
The largest published single center experience is from the University of Michigan which reported 77 % survival in 128 patients between 1985 and 1998 (Swaniker et al. 2000). The mean duration of support in this series was 12 ± 10 days (median 9.5) with a range of 0.2–47 days. A similar outcome was reported from Atlanta in 63 patients between 1991 and 2002 (Pettignano et al. 2003). The underlying disease process has an impact on outcome with the highest survival in patients with viral pneumonia in the ELSO Registry. Within this category, Moler et al. (1993) reported a 49 % survival of patients with RSV, although this series was published more than 10 years ago since ventilation practices have changed substantially. A more recent series of ex-premature infants with RSV showed an 80 % survival to hospital discharge, but with a 60 % neurodisability rate (Brown et al. 2004). Good outcomes have been reported in small case series for pulmonary hemorrhage (Kolovos et al. 2002; Siden et al. 1994), burns and smoke inhalation (Goretsky et al. 1995; Kane et al. 1999; Leesin et al. 1996; Pierre et al. 1998), posttraumatic lung injury (Fortenberry et al. 2003), and occasional patients with severe asthma and hypercarbia (Hebbar et al. 2009). Two disease categories remain particularly challenging. AHRF secondary to pertussis that presents in the first 3 months of life is associated with a particularly severe form of pulmonary hypertension which does not resolve. The mortality in these patients even with ECMO support is 80 % (Halasa et al. 2003; Pooboni et al. 2003). The second group is those patients with hematological malignancies. Data from the ELSO Registry on patients placed on ECMO following bone marrow transplantation published up to 2006 showed only one survivor to hospital discharge in 19 patients (Gow et al. 2006). For children with immunosuppression, the mortality is higher than in immunocompetent patients reported to ELSO (Gupta et al. 2008) but there are some small case series with good outcomes. Linden reported 3/4 children with pneumocystis pneumonia surviving (Linden et al. 1999) and 2/4 survivors in children with leukemia and ARDS in a second series (Meister et al. 2010). As can be anticipated the cost of ECMO in financial terms is significant. Vats (Vats et al. 1998) has computed the hospital costs to be $200,000 which translates into $4,190/life-year.
The efficacy of ECMO as a lifesaving therapy in neonatal AHRF has a sound basis supported by a high level of evidence. The case is more difficult to make in older children in the absence of any randomized trial evidence. The only attempt at such a study was abandoned because with a change in ventilation practice, the predicted mortality was substantially less than the actual (Fackler et al. 1997). There is however a large amount of evidence from case series and Registry data which supports its use. In a multi-institutional retrospective cohort analysis of over 300 patients with AHRF from 32 centers over a 1-year period in an era before the use of lung-protective ventilation, ECMO was associated with reduced mortality in the group of patients with the highest OI compared with those that received conventional or high-frequency ventilation (Green et al. 1996). However, even in an era of lung-protective ventilation and widespread use of high-frequency ventilation, children who are impossible to oxygenate can be saved by having access to ECMO. This was illustrated in the 2009–2010 H1N1 epidemic where 103 patients <20 years of age were put on ECMO with a 48 % survival (http://www.elso.umich.edu/H1N1Registry). The question arises as to what defines a failure of conventional therapy as a prelude to ECMO. Options will be influenced by published literature but also by physician bias. Figure 66.3 provides an outline of an algorithm for clinical decision making in such a scenario.
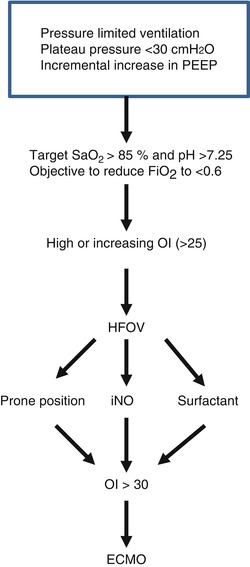

Fig. 66.3
Algorithm for severe AHRF
66.6 Extracorporeal Pulmonary Support: Future Directions
The major advances in technology that have occurred in the past 20 years have led to innovative thinking of the application of extracorporeal support in severe AHRF. The compact design of pumps and oxygenators has made transport on ECMO a reality rather than a novel event that requires prolonged planning and major consumption of resources (Clement et al. 2010; Prodhan et al. 2010; Coppola et al. 2008). This notwithstanding, ECMO is a complex, labor-intensive system with attendant risks. One of the newer innovations has been the introduction of a pumpless system for extracorporeal CO2 removal in patients with AHRF where hypercarbia is a feature. Considerable experience has now accumulated with the Novalung where patients are cannulated by A-V access through femoral artery and vein, and the device is perfused by systemic pressure with sufficient flow to remove CO2 and partially support oxygenation (Florchinger et al. 2008; Zimmermann et al. 2009; Weber-Carstens et al. 2009). In situations where flow is insufficient, a pump can be added to the circuit. Pediatric experience is this device is limited so far, but there are case reports of it being used successfully in patients with asthma and severe hypercarbia (Romano 2007; Conrad et al. 2007). It is being used increasingly as a bridge to lung transplantation in patients with cystic fibrosis and primary pulmonary hypertension (Fischer et al. 2007; Ricci et al. 2010). In this situation hypercarbia is often associated with such severe right ventricular dysfunction and limitation of pulmonary blood flow that effective removal of CO2 can only be achieved with transpulmonary placement of the device (pulmonary artery to pulmonary vein) (Strueber et al. 2009).
Essentials to Remember
ECMO is the ultimate high-tech modality for the support of respiratory failure in critical care. While the technology has improved significantly in the past 20 years, the survival outside the newborn period is still only 50 % and the complications are significant.
Pulmonary failure is usually a single-system disease in the newborn, while it is frequently part of a multiorgan dysfunction syndrome in older children. Hence, the outcomes will be less good.
As technology improves and the complication rate falls, more children previously deemed ineligible will be considered for ECMO.
Pumpless extracorporeal CO2 removal is a very useful modality as a bridge to transplantation in patients with severe hypercarbia due to pulmonary vascular disease and right heart dysfunction.
References
Baffes TG, Fridman JL, Bicoff JP, Whitehill JL (1970) Extracorporeal circulation for support of palliative cardiac surgery in infants. Ann Thorac Surg 10(4):354–363PubMedCrossRef
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree