Esophageal Atresia and Tracheoesophageal Fistula Malformations
Esophageal atresia (EA) and tracheoesophageal fistula (TEF) anomalies present the pediatric surgeon with a unique and complex congenital disease, which tests both the diagnostic and technical skill of the surgeon. Most pediatric surgeons consider the surgical correction of these malformations to be the height of neonatal surgical care. In 1959, Dr Willis Potts wrote, ‘To anastomose the ends of an infant’s esophagus, the surgeon must be as delicate and precise as a skilled watchmaker. No other operation offers a greater opportunity for pure technical artistry.’1 While this statement still remains true, improvements in anesthetic and neonatal intensive care have made repair of these anomalies and their postoperative management much more routine so that a good outcome can be achieved in most cases. Technical advances, including the application of the minimally invasive approach, have also decreased the morbidity from these operations.
The first report of EA was by Durston in 1670,2 who found a blind upper pouch in one of a pair of thoracopagus conjoined twins, but the initial classic description was by Thomas Gibson in 1697.3 However, it was not until 1939 when a baby with EA/TEF survived following successful staged repairs described separately by Leven and Ladd.4,5 In 1940, Haight described the first survival following primary anastomosis.6 By the mid-1980s, most neonatal centers were performing primary repair and reporting successful outcomes in up to 90%.7–10
Embryology
The embryology of the foregut is still subject to controversy.11 What is known, however, is that during the fourth week of gestation the foregut starts to differentiate into a ventral respiratory part and a dorsal esophageal part. The laryngotracheal diverticulum then invaginates ventrally into the mesenchyme. The traditional theory postulates that the ventral respiratory system separates from the esophagus by the formation of lateral tracheoesophageal folds that fuse in the midline and create the tracheoesophageal septum. At 6 to 7 weeks of gestation, the separation between trachea and esophagus is complete. Incomplete fusion of the folds results in a defective tracheoesophageal septum and abnormal connection between the trachea and esophagus.
This theory of longitudinal tracheoesophageal folds merging to form a septum has been challenged.12,13 In chick embryo studies, these folds could not be demonstrated. Instead, cranial and caudal folds were found in the region of tracheoesophageal separation. According to this theory, EA/TEF would then be due to an imbalance in the growth of these folds. Furthermore, rat studies suggest that EA/TEF results from disturbances in either epithelial proliferation or apoptosis.14
More recent studies show that ectopic expression of sonic hedgehog occurs in the tissues between the notochord and the gut. Knockout mice models have helped elucidate the functions of different genes in the development of the foregut aberrations such as EA/TEF.15 Also, the relationship between BMP4 (bone morphogenic protein) and Nog, the gene encoding noggin (which is a BMP antagonist), may also have an impact on the development of TEF.16–18
Epidemiology
The birth incidence of EA/TEF varies between 1 in 2500 to 3000 live births.19–21 There is a slight male preponderance of 1.26 : 1. There is no evidence for a link between EA/TEF and maternal age when chromosomal cases are excluded.22 The risk for a second child with EA/TEF among parents of one affected child is 0.5–2%, increasing to 20% when more than one child is affected. The empirical risk of an affected child born to an affected person is 3–4%.23 The relative risk for EA/TEF in twins is 2.56 when compared with singletons.24 The concordance rate in twins is low, but the risk among twins of the same gender is high.25
Environmental factors that have been implicated include the use of methimazole in early pregnancy, prolonged use of contraceptive pills, progesterone and estrogen exposure, maternal diabetes, and thalidomide exposure.26–30 EA is occasionally seen in the fetal alcohol syndrome and in maternal phenylketonuria.31,32
Chromosomal anomalies are found in 6–10% of the patients.33–35 The total number of trisomy 18 cases exceeds the total number of trisomy 21 cases. As the incidence of trisomy 18 is higher, it would seem to indicate that trisomy 18 is a greater risk for EA development. Three separate genes have been associated with EA/TEF: MYCN haploinsufficiency in Feingold syndrome, CHD7 in CHARGE syndrome, and SOX2 in the anophthalmia–esophageal–genital (AEG) syndrome.34–37
EA may occasionally be part of the Opitz G/BB syndrome, Fanconi anemia, oculo-auriculo-vertebral syndrome, Bartsocas–Papas syndrome, or Frijns syndrome.38
Associated Anomalies
The factor or factors responsible for the early disturbance in organogenesis causing EA may affect other organs or systems that are developing at the same time. EA can be divided clinically into isolated EA and syndromic EA, occurring at roughly the same rate.38
The most frequent associated malformations encountered in syndromic EA are:
Vertebral anomalies are confined mainly to the thoracic region. An earlier claim that the presence of 13 pairs of ribs is a good indicator of long-gap EA has not been substantiated.39
Nonrandom associations have been documented as well. Two of these are the VACTERL association (vertebral, anorectal, cardiac, tracheo-esophageal, renal, and limb abnormalities) and the CHARGE association (coloboma, heart defects, atresia of the choanae, developmental retardation, genital hypoplasia, and ear deformities). In 1973, VACTERL was originally described as VATER, an acronym made up of vertebral defects, anal atresia, tracheo-esophageal fistula with EA, and radial dysplasia.40 It was later extended with the C for cardiac anomalies and the L for limb anomalies. In a cohort of 463 patients with EA, 107 (23%) had at least two additional VACTERL defects.41 Seventeen of these patients had a chromosomal defect or a syndrome without a known genetic defect. Interestingly, as many as 70% of the remaining 90 patients had additional defects other than VACTERL anomalies.
Classification
EA and TEF present in many forms and various classification systems have been used to describe them. It is clear that EA should be thought of as a spectrum of anomalies (Fig. 27-1).42 The original classification system was devised by Vogt in 1929.43 Ladd put forth his own classification in 19455 and Gross revised this in 1953.44 These classifications tend to be confusing, as the same subclasses are named differently. For clarity, it seems much better to give descriptive names to the major subtypes.
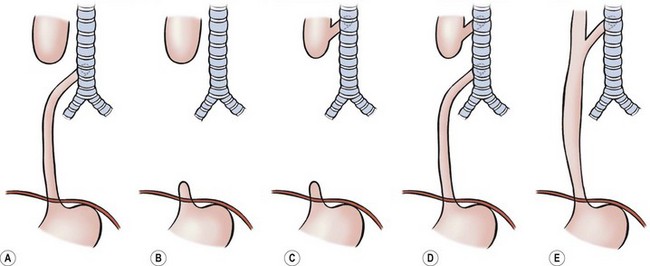
FIGURE 27-1 Classification of EA and/or TEF: (A) esophageal atresia with distal tracheoesophageal fistula: Vogt IIIb, Ladd III, Gross C; (B) esophageal atresia without fistula: Vogt II, Ladd I, Gross A; (C) esophageal atresia with proximal fistula: Vogt IIIa, Ladd II, Gross B; (D) esophageal atresia with proximal and distal fistulas: Vogt IIIc, Ladd V, Gross D; (E) tracheoesophageal fistula (H-type) without atresia: Vogt IV, Gross E.
Esophageal Atresia with Distal Fistula (Gross Type C)
This is the most common subtype, accounting for about 85% of EA anomalies.45 The very dilated proximal esophagus has a thickened wall and descends into the superior mediastinum usually to the third or fourth thoracic vertebrae. The distal esophagus is slender and has a thin wall. It enters the trachea posteriorly either at the level of the carina or 1–2 cm higher. The distance between the esophageal ends varies from very small to quite wide. Very rarely, the distal fistula may be occluded, leading to the misdiagnosis of EA without distal fistula.46
Pure Esophageal Atresia without TEF (Gross Type A)
Pure EA has an incidence of about 7%. The proximal and distal esophagus end blindly in the posterior mediastinum. The proximal end is dilated and has a thickened wall as in the more common EA/TEF. If there is no concomitant proximal fistula, the upper esophagus ends at the level of the azygos vein. The distal esophagus is short and often suspended by a fibrotic band. The distance between the two segments is considerable, usually precluding immediate anastomosis.
H-type Fistula without Esophageal Atresia (Gross Type E)
H-type TEF without atresia is usually discussed together with EA because it may be part of the VACTERL association. It occurs with an incidence of about 4%. The fistula starts from the membranous trachea and runs caudad to enter the esophagus. Normally it is short, although the diameter may be variable. The fistula is usually situated at the thoracic aperture or higher in the neck.47
Esophageal Atresia with Proximal Fistula (Gross Type B)
The association of a proximal fistula in a patient with pure EA is generally thought to be about 2%, but may be higher than is generally appreciated. In a recent series of 13 children without distal fistula, a proximal fistula was found in seven.48 An upper esophageal fistula is usually not found at the end of the pouch. This fistula is similar to the H-type starting proximally on the trachea and ending distally in the dilated proximal esophagus. Usually, there is only one proximal fistula, but two or three have been described.49 The fistula is usually located at the thoracic aperture or higher in the neck. Although limited in length, its diameter may vary from tiny to large. If not diagnosed preoperatively, it may be suspected during operative repair when bubbles are seen when opening the proximal esophagus.
Esophageal Atresia with Proximal and Distal Fistulas (Gross Type D)
The incidence of EA with proximal and distal fistulas is thought to be less than 1%. EA with one distal fistula and two proximal fistulas has also been described.49 Also reported is a near-complete membranous obstruction of the esophagus in conjunction with a single TEF at the level of the membrane, communicating with both parts of the esophagus.50
Diagnosis
Antenatal Diagnosis
The prenatal diagnosis of EA/TEF relies, in principle, on two nonspecific signs: polyhydramnios and an absent or small stomach bubble. Polyhydramnios is associated with a wide range of fetal abnormalities and is nonspecific. Similarly, the ultrasonographic (US) absence of a stomach bubble may point to a variety of fetal anomalies. The combination of a small stomach together with a dilated cervical esophagus (the pouch sign) has been confirmed to be diagnostic for pure EA in a number of patients.51–53 Nevertheless, it is encountered in only a few patients. Current ultrasound technology does not allow for the certain diagnosis of EA/TEF. Therefore, definitive counseling of the parents should be guarded.54 The application of 3D power Doppler imaging seems promising, both antenatally and postnatally. Aortic arch anomalies, for example, have been diagnosed using this modality.55,56
Magnetic resonance imaging (MRI) has been used to identify other fetal thoracic lesions, and may be beneficial in patients deemed to be at risk on prenatal ultrasound. Sensitivity in various studies is between 60–100% and the diagnosis is made by the lack of visualization of the thoracic esophagus.57–59
Postnatal Diagnosis
As EA prevents the passage of saliva down the esophagus, saliva accumulates in the proximal esophagus and mouth, and feeding should be withheld until esophageal continuity is confirmed. This is best done with a stiff 10 French catheter inserted either through the nose or mouth. A chest film is then obtained with downward pressure on the tube. With EA, the tip of the tube is found to be slightly curled in the blind upper pouch around T2–T4 (Fig. 27-2). This technique not only identifies the atresia but gives some clue to the length of the upper pouch. Often, the dilated upper esophageal pouch is visualized by air within it. Air in the stomach signifies the presence of a distal TEF. If the tip of the catheter passes beyond the level of the carina, then the diagnosis of EA should be questioned. Esophageal stenosis, tracheal rings, and iatrogenic perforation of the esophagus can be confused with EA.60,61 If there is any question about the diagnosis, a small amount of contrast can be dripped into the upper pouch, but this needs to be done with fluoroscopy and under direction of the surgeon to assure that the contrast is not aspirated.
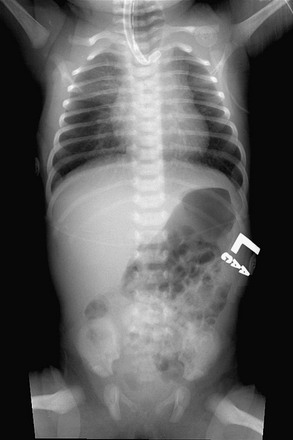
FIGURE 27-2 This plain radiograph depicts the classic features seen in an infant with EA and TEF. A nasoesophageal tube is seen in the upper pouch and has kinked a little at the end of the pouch. There is air in the stomach and bowel, which signifies the presence of a distal TEF.
Radiographs may reveal associated anomalies such as vertebral and rib anomalies, or other problems such as duodenal atresia (Fig. 27-3). The absence of air in the stomach points to EA without distal fistula (see Fig. 27-3A). Mediastinal ultrasound has been suggested as a helpful adjunct in the diagnosis of pure EA.62
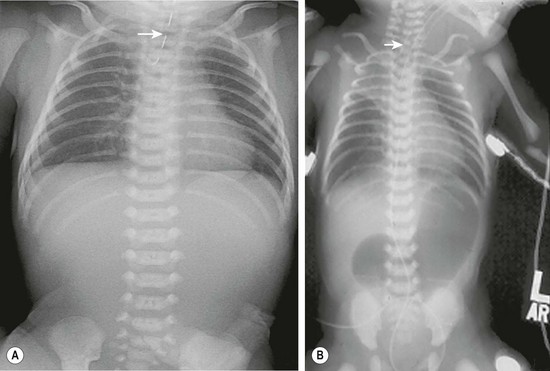
FIGURE 27-3 These two radiographs depict less common presentations of EA. (A) Isolated EA. The nasoesophageal tube (arrow) is seen in the proximal pouch. There is no air in the gastrointestinal tract. (B) This patient has EA with distal TEF and duodenal atresia and has been endotracheally intubated. A nasoesophageal catheter (arrow) sits in the upper esophageal pouch. An endotracheal tube is also seen. The stomach and bulbous duodenum are distended with air but no air is seen distal to the duodenum.
The length of the esophageal gap is usually not known preoperatively. Absence of air in the stomach has been linked with a long gap, but has also been described in association with a distal fistula occluded with mucus.46 Even in true long-gap EA (atresia without distal fistula), the gap length varies. In newborns with isolated EA, the first procedure is generally a gastrostomy, which allows for enteral feeding and also allows for assessment of the length and location of the lower pouch. It can be identified radiologically, either with metal bougies, a small gastroscope, or with a contrast injected through the gastrosomy.63 This can be done at the time of the initial gastrostomy or more routinely seven to ten days later. If bougies or a telescope are introduced into the distal esophagus, the amount of pressure on these instruments will affect the measurement of the gap between the two esophageal segments and may under- or over-estimate the gap length. In one report by an experienced surgeon, operative management was linked to the measured gap length: less than two vertebrae, then primary anastomosis; two to six vertebrae, then delayed primary anastomosis; more than six vertebrae, then esophageal replacement.64 In the era of thoracoscopy, an initial thoracic exploration can be considered if the upper pouch appears fairly long. If the lower pouch is identified and seen to be of adequate length, a primary repair can be attempted. If not, then a gastrostomy can be placed and delayed repair planned.65
In EA/TEF, a longer gap between the two esophageal ends should be expected when the distal fistula is found at the carina.66 Combined with a short upper pouch, this can mean a long gap exists between the two esophageal segments and may not be amenable to an initial primary repair. Unfortunately this may not fully be appreciated until the time of exploration. However since it is usually necessary to ligate the fistula in the early postnatal period, the gap length can be assessed at that time. A thoracoscopic approach in this scenario allows for minimal morbidity if the decision is to ligate the fistula only without reconstruction. Bronchoscopy can also be performed prior to exploration, not only to assess the site of the distal fistula, but to also look for an upper pouch fistula. Echocardiography should be performed prior to operation as it may reveal cardiac and/or aortic arch anomalies. A right descending aorta, which occurs in about 2.5% of the cases, may make a left-sided thoracic approach preferable.67 Renal ultrasound and spine radiographs should be obtained as well. Because EA may be part of a syndrome, consultation by a geneticist is recommended at some point.
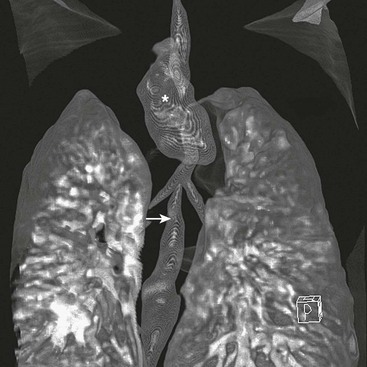
There is little doubt that better preoperative imaging of the neck and chest allows for better preoperative planning. At present, the two best imaging modalities are CT and MRI. Although MRI would certainly be preferable for its absence of radiation exposure, it requires general anesthesia. On the other hand, MRI is better for diagnosing cardiac and aortic arch anomalies.68 CT has also been performed in children with EA/TEF (Fig. 27-4).69–72
Management
Preoperative
Once the diagnosis of EA has been established, the baby, if not delivered in a maternal/fetal/neonatal center, should be transferred to a pediatric surgical center. A 10 French Replogle tube is placed in the upper esophagus and set to continuous suction.73 The child is positioned head-up and on his or her side. Intravenous access is important, and the vital signs are monitored.
If the child is in respiratory distress, endotracheal intubation and ventilation may be needed. Forceful ventilation will over distend the stomach, possibly causing diaphragmatic splinting and even gastric rupture.74,75 Gentle low-pressure ventilation is therefore essential. In these cases, some feel high-frequency ventilation is advantageous. However, on occasion, emergency ligation of the fistula may be needed as a life-saving maneuver.64,74–76 After ligation of the fistula, a delayed primary repair can be performed when the infant is more stable. Some recommend not postponing longer than seven to 14 days because recanalization of the fistula can occur.64,77,78 In these patients, we have performed thoracoscopic fistula ligation followed by repair two to six weeks later. We have not seen recanalization in our experience. If the infant is extremely unstable, then an emergency gastrostomy to decompress the stomach may be the best option. However this can result in a significant loss of tidal volume and may create respiratory issues as well.
Operative Repair
Esophageal Atresia with Distal Fistula
The operation is performed with the patient under general anesthesia and with adequate venous access. An arterial line is occasionally beneficial depending on the baby’s clinical status. Generally, a pulse oximeter and end tidal CO2 monitor are adequate. The operation can be performed through a thoracotomy or using a thoracoscopic approach. The side of entrance into the chest is opposite the turn of the aortic arch: right for a left descending aorta, left for a right descending aorta. If a right-sided aortic arch is not detected until the operation has begun, change to the left side is appropriate if the thoracoscopic approach was initially chosen. If a thoracotomy has been performed, an anastomosis from the right chest should be attempted, but there is a higher morbidity in this setting.67
Preoperative Bronchoscopy
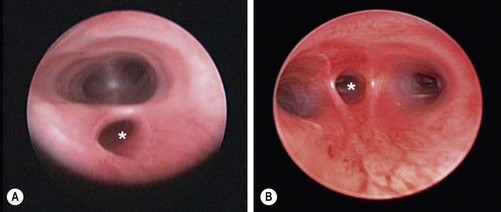
The value of routine preoperative rigid bronchoscopy is much debated because the incidence of a simultaneous proximal and distal fistula is less than 1%.63 With the availability of small-diameter flexible fiberscopes, tracheobronchoscopy can now be performed after intubation through the endotracheal tube.79,80 Forceful ventilation must be avoided, not only to avoid lung damage,81 but also to prevent gastric distention and gastric perforation with insufflation through the distal fistula. Bronchoscopy may reveal abnormalities such as a second proximal fistula, a laryngotracheoesophageal cleft, tracheal stenosis, or a tracheal bronchus to the right upper lobe.82–84 The entrance of the distal fistula is usually well seen (Fig. 27-5). Its distance to the carina provides a clue as to the gap between the esophageal segments: the closer the fistula is to the carina, the longer the distance. An indication of the severity of tracheomalacia is only possible when the child is breathing spontaneously. The problem with bronchoscopy is that it may prolong the procedure and the infant can decompensate prior to ligation of the fistula.85–88 The surgeon and the anesthesiologist should discuss the advantages/disadvantages in each individual case. Following intubation, it may be helpful to position the endotracheal tube distal to the fistula, assuming the fistula is not at the carina.
Repair via Thoracotomy
The patient’s arm is positioned over the head (Fig. 27-6). Suction is removed from the Replogle tube, but the tube is left in place so that it can be advanced during the operation to aid in identifying the proximal pouch.
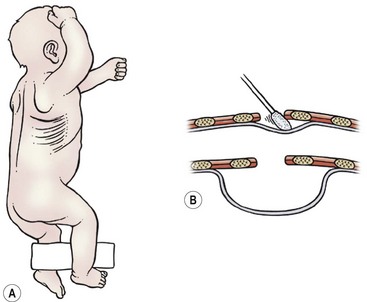
FIGURE 27-6 (A) The infant is positioned for a right thoracotomy. The ipsilateral arm is positioned over the head of the patient. A 4–5 cm incision is made 1 cm below the tip of the scapula. (B) This schematic depicts a peanut or sterile cotton swab being used to gently push the pleura away from the chest wall.
A slightly curved 4–5 cm long incision is made 1 cm below the inferior tip of the scapula. With the use of a muscle-sparing approach, the auscultatory triangle is opened and the muscles are retracted (i.e., the latissimus dorsi posteriorly and the serratus anterior anteriorly).89,90 If the serratus muscle needs to be transected, this should be done as low as possible to preserve the long thoracic nerve. The fourth or fifth intercostal space is then entered.
An extrapleural approach has been suggested to protect the pleural space in case of an anastomotic leak, but there is no evidence that it is better than a transpleural one.91,92 However an extrapleural approach does aid in exposure as it is easier to retract the lung when it is incased in the pleura. With the extrapleural approach, the pleura is gently pushed away from the endothoracic fascia, first in the middle of the incision so that an infant rib spreader can be inserted and opened (see Fig. 27-6B). With the rib spreader opened even farther, the pleura is carefully pushed away posteriorly until the posterior mediastinum is exposed.
The distal fistula may start from the trachea directly underneath the azygos vein, in which case the azygos vein is transected between 3-0 or 4-0 absorbable ligatures or simply cauterized and divided (Fig. 27-7). If the distal fistula originates more cephalad on the trachea, the vein can be left intact. Recently, a relationship between azygos vein transection and anastomotic leak has been suggested.93,94
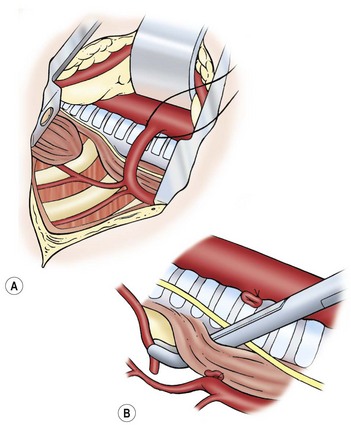
FIGURE 27-7 (A) Ligation of the azygos vein. (B) The distal fistula is being mobilized from its insertion in the trachea.
The distal esophagus is easily found because it distends with each inspiration and the vagus nerve is intimately attached. Once identified, the distal segment should be followed proximally to locate where the fistula enters the trachea. The fistula should be dissected and mobilized close to the trachea, which will spare as many vagal nerve branches as possible (see Fig. 27-7B). The fistula can be encircled with a vessel loop or suture to aid in exposure. There are several ways to ligate the fistula on the tracheal side. Ligation in continuity can result in a higher recanalization rate.95,96 We prefer to divide the fistula sharply after placing traction sutures at each end. A series of 5-0 PDS (Ethicon, Inc, Sommerville NJ) sutures are then used to close the tracheal side taking care not to compromise the tracheal lumen (Fig. 27-8). Another option is to apply a single 5mm clip across the fistula where it connects to the membranous trachea. We have found this technique to be simple and efficient, and results in a smaller pouch remnant on the posterior tracheal wall. However, there are anecdotal reports of clip migration using this technique. The tracheal closure can be checked by irrigating with warm water and applying a higher ventilation pressure to assess for an air leak.
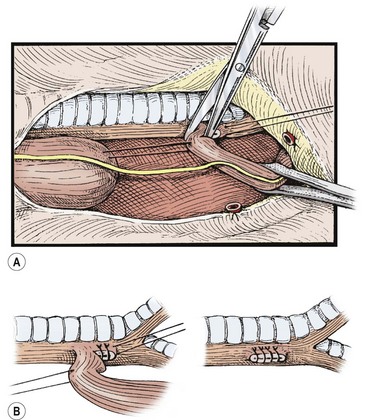
FIGURE 27-8 The distal esophagus is identified, looped, and carefully dissected up to its junction with the trachea, meticulously sparing the segmental vessels from the aorta. (A) Traction sutures may be placed for gentle handling of the segments. The fistula is divided close to the trachea without narrowing its lumen. (B) The tracheal end of the tracheoesophageal fistula is closed with continuous or interrupted sutures. Adjacent tissue, if available, is tacked over the closure. (From Holder TM, Manning PB. Esophageal atresia and tracheoesophageal fistula. Surg Rounds 1991;14:492–502.)
Attention is then turned to the proximal esophagus which can be identified by asking the anesthesiologist to push on the Replogle tube. A traction suture, taking a good bite of the muscular wall, is placed in the most distal part of the proximal pouch. Using the traction suture, the proximal pouch can be freed posteriorly and laterally by blunt dissection. Anteriorly, however, the pouch may be adherent to the membranous trachea. Usually, it can be dissected sharply, staying on the esophageal side to avoid entrance into the membranos trachea. Extensive dissection is not warranted, unless there is a long gap, because the dissection can damage the tracheal or esophageal walls and may interfere with innervation to the upper esophagus.97 Extensive dissection of the proximal pouch searching for a proximal fistula should not be performed routinely because the incidence of a proximal fistula in combination with a distal one is only about 1%. If a proximal fistula is present, it is usually of the H-type. If missed at birth and diagnosed after repair of the EA, it can usually be repaired through the neck at a later date. If the trachea is entered during the dissection of the proximal pouch, one should be wary that a proximal fistula may have been opened as well. There should not be a common wall between the trachea and esophagus.
After mobilization of the proximal pouch, the tip is now amputated so that the lumen and mucosa become visible. An end-to-end anastomosis is performed with 5-0 absorbable sutures starting in the middle of the back wall of each esophageal segment (Fig. 27-9). It is important to include both the mucosa and the muscular wall with each suture. The sutures in the back wall of the anastomosis are tied intralumenally. Then, the front part of the anastomosis is performed with sutures tied on the outside. Before finishing the anastomosis, an 8 French or 10 French tube is passed into the stomach. This protects from inadvertent closure of the lumen and for gastric decompression. Others feel the placement of an intralumenal tube or stent causes an increased risk of stricture or leak, and choose not to use it.
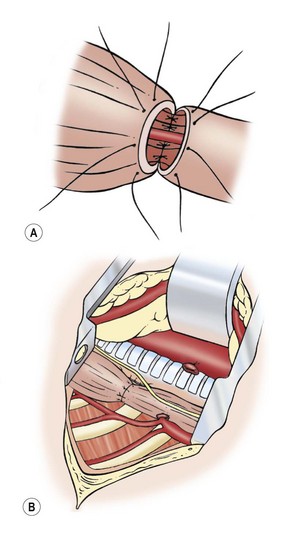
FIGURE 27-9 Esophageal anastomosis. (A) The back wall of the anastomosis has been sutured from the inside, and a small nasogastric tube has been passed through the anastomosis. The front part of the anastomosis is being sutured with knots on the outside. (B) Completed anastomosis.
The use of a chest drain is also optional.98,99 We use one until a contrast study is obtained on day 4 or 5. The chest incision is then closed in layers. The ribs should be approximated with one 3-0 absorbable suture. This suture should be tied gently so that the intercostal space is not obliterated. If a muscle-sparing approach was used, the muscles are allowed to fall back into their normal position and the skin is closed with a 5-0 absorbable subcuticular suture.
Thoracoscopic Repair
The first successful repair was reported in 2000 and the first series reported in 2002.100,101 Since then, there have been several retrospective reports describing experience with the thoracoscopic approach.102–105 During this early experience, attempts were made to obtain single lung ventilation by intubating the left mainstem bronchus. However, this proved time consuming and often unsuccessful so now the endotracheal tube is usually left in the trachea just above the carina and right lung collapse is achieved with CO2 insufflation alone.106 Others have described using the oscillating ventilator to effect lung collapse and negate the adverse effects of prolonged hypercarbia.107
Positioning.
Once the endotracheal tube is secure, the patient is placed in a modified prone position with the right side elevated approximately 30° (Fig. 27-10). The patient is placed near the edge of the table so that the handles of the instruments do not collide with the table. (If there is a right-sided arch, then a left-sided approach is used.) This positioning gives the surgeon access to the area between the anterior and posterior axillary lines for port placement while allowing gravity to retract the lung away from the posterior mediastinum. This arrangement allows excellent exposure of the fistula and esophageal segments without the need for an extra instrument or lung retractor. The assistant should not be placed on the opposite side of the table as this will place him/her at a complete paradox with the telescope. The scrub nurse can be on either side of the baby depending on the room layout. Because of the fine manipulation necessary, the surgeon and the assistant should position themselves so that they are in the most ergonomic and comfortable position.
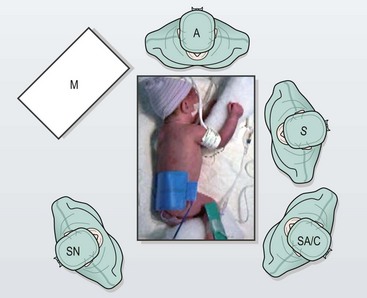
FIGURE 27-10 This infant is positioned more prone than lateral for right thoracoscopic repair. The surgeon (S) stands on the left side of the operating table when the aortic arch turns to the left. The surgeon, operative field, and screen are in-line. The assistant and camera holder (SA/C) is situated to the left of the surgeon when the surgeon is right-handed. The scrub nurse (SN) stands to the right of the operating table. M, monitor; A, anesthesiologist. (From Holcomb GW, Rothenberg SS, Georgeson KE. Atlas of Pediatric Laparoscopy and Thoracoscopy. Elsevier; 2009.)
Port placement is extremely important because of the small chest cavity and the intricate nature of the dissection and reconstruction. Usually three ports are satisfactory, but a fourth one can be used if needed (Fig. 27-11).
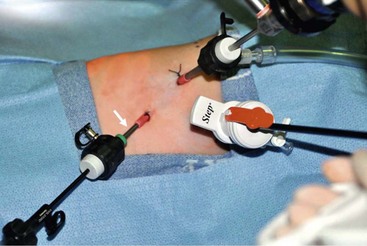
FIGURE 27-11 Optimal port positions for a thoracoscopic repair are seen. The ports are inserted in triangulation to allow the working instruments to meet at 90°. The camera port is inserted just below and posterior to the tip of the scapula. It is also the site for insufflation. One working port (Step, in photograph) is inserted in the midaxillary line in the axilla. The second working port (arrow) is introduced in the posterior axillary line. (From Holcomb GW, Rothenberg SS, Georgeson KE. Atlas of Pediatric Laparoscopy and Thoracoscopy. Elsevier; 2009.)
The initial port (3 mm or 4 mm) is placed in the fifth intercostal space behind the tip of the scapula. This is the telescope and camera port and allows excellent visualization of the posterior mediastinum. An angled telescope (30° or 45°) is essential. The two instrument ports are then introduced. The first is in the midaxillary line one or two interspaces above the telescope port in the axilla. This cephalad port is 5 mm for the clip applier and needle driver/suture. The lower port is 3 mm and is located in the posterior axillary line two interspaces below the telescope port. Ideally, these ports are positioned so that the instrument tips will approximate a 90° angle at the level of the fistula which will facilitate performing the anastomosis.
With the vein divided, the lower esophageal segment is identified and followed proximally to the fistula. Because of the magnification afforded by the thoracoscopic approach, it is easy to visualize exactly where the distal fistula enters the back wall of the trachea (Fig. 27-12
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree