GINI FLEMING  JEFFREY SEIDMAN
JEFFREY SEIDMAN  ERNST LENGYEL
ERNST LENGYEL
EPIDEMIOLOGY AND RISK FACTORS
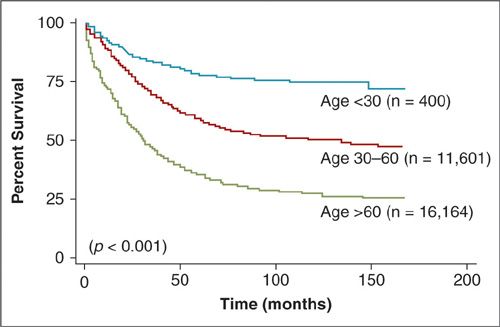
Established risk factors for ovarian cancer include a strong family history, early menarche, late menopause, increasing age, and nulliparity. The most important risk factor, after having a first-degree relative with the disease, is age. Women younger than 40 years without a positive family history rarely have ovarian cancer. Fifty percent of all cases of ovarian cancer in the United States occur in women over the age of 65. Older women have a much worse prognosis overall (Fig. 24.1) in part because they have an increased incidence of high-stage and high-grade disease at the time of diagnosis (Table 24.1) (1, 2). However, in an analysis of the The Surveillance, Epidemiology, and End Results (SEER) database, age remained a poor prognostic factor even when results were adjusted for stage, grade, histologic cell type, race, and surgical treatment. The relative risks (RR) associated with endocrinologic factors are much smaller, though important, because they are potentially subject to modulation.
Epidemiology
Epithelial ovarian cancer is the leading cause of death from gynecologic cancer in the United States and Europe (excluding breast cancer). Data from the American Cancer Society suggested that 22,280 new cases of ovarian cancer and 15,500 deaths from ovarian cancer would be expected in the United States in 2012 (3). It has been estimated that, in the United States, 1 woman in 70 will develop ovarian cancer, and 1 woman in 100 will die of the disease. In Europe, the International Agency for Research on Cancer in Lyon estimated that in the 40 European reporting countries, there will be 66,734 new patients with ovarian cancer and 41,929 deaths (4). Both in the United States and in Europe, ovarian cancer is the fifth most common cause of cancer death. Estimates for global ovarian cancer burden, which include low malignant potential cancers (the U.S. and European numbers exclude it), are that 225,500 patients will develop epithelial ovarian cancer and about 140,200 will succumb to the disease.
Ovarian cancer rates vary between different countries and appear to be linked to socioeconomic status and reproductive patterns. North America and most of the industrialized countries of Europe have high incidence rates, while the disease is rare in Asia and Africa. The lowest rates of ovarian cancer are in Sub-Saharan Africa (Fig. 24.2). While the reason for this difference has not been elucidated, countries with a high incidence rate are generally characterized by smaller family sizes, high-fat diets, higher socioeconomic status, older age, and a predominantly Caucasian population. Once a woman moves from a country with a low incidence of ovarian cancer to one with a high incidence, her risk for the disease tends to approach that of the adopted country rather than the country of origin.
Weight/Body Mass Index
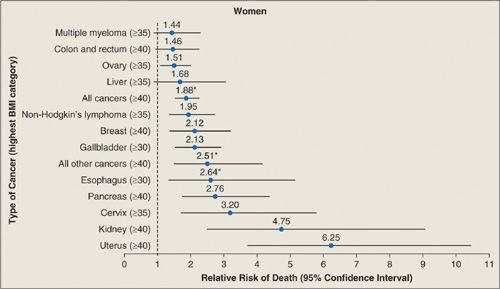
Many epidemiologic studies have reported that height and weight are relevant to a woman’s risk of developing ovarian cancer, although the findings of these studies have been inconsistent. In a prospective cohort study that followed 495,477 women for 6 years, body mass index (BMI) and ovarian cancer mortality were significantly associated (Fig. 24.3). For women with a BMI of 35 to 39, the relative risk of developing ovarian cancer was 1.5 (CI, 1.1–2) while the relative risk of developing endometrial cancer was 6.3 (CI, 3.8–10.4). In 2012, a meta-analysis that summarized 47 studies involving 25,157 women with ovarian cancer and 81,311 women without the disease found a significant increase in relative risk (RR, 1.07) of ovarian cancer per 5-cm increase in height. The relative risk for ovarian cancer per 5 kg/m2 increase in BMI was 1.1 in women who did not take hormone replacement therapy (HRT) but only 0.95 in women on HRT (6). The relationship between ovarian cancer risk and a high BMI at age 18 was stronger than the relationship between ovarian cancer risk and a high BMI occurring later in life (7). In summary, there is probably a small (1.3) but significant increase in the odds ratio for developing ovarian cancer if a woman’s BMI is more than 30 (5, 8). Given that the association of obesity and ovarian cancer is not strong, it is possible that it is caused by other confounding factors such as type 2 diabetes, high-fat diets, or factors that are currently unknown (9).
Reproductive Factors: Pregnancy and Breast Feeding
Early menarche and late menopause increase the risk of ovarian cancer, while increased parity, breastfeeding, and the use of oral contraceptives (OCP) reduce the risk. These findings suggest that the risk for ovarian cancer is linked to the number of ovulations that a woman experiences throughout her lifetime.
Several epidemiologic studies clearly show that pregnancy, breast-feeding, and OCP use are associated with a reduced risk of developing ovarian cancer (10 – 12). In addition, a number of case-control studies have shown that pregnancy lowers ovarian cancer risk and that the risk reduction is higher with each additional pregnancy. Interestingly, a pregnancy after age 35 is more protective against ovarian cancer than a pregnancy in a woman 25 years or younger (13).
In general, breast-feeding reduces the risk of ovarian cancer: Women who breast-feed for longer than 12 months have a substantial reduction in the risk of ovarian cancer, which is in addition to the risk reduction derived from childbirth (14). Breast-feeding probably reduces ovarian cancer incidence through several mechanisms, including suppression of ovulation, reduced serum concentrations of estradiol and LH, and elevated FSH levels.
FIGURE 24.1. Disease-specific survival of patients based on age at diagnosis.
Source: Reprinted with Permission from Chan JK, Urban R, Cheung MK, et al. Ovarian cancer in younger vs. older women: a population-based analysis. Br J Cancer. 2006;95:1314–1320.
Oral contraceptives
Women who use OCP for at least 5 years reduce their risk of ovarian cancer by an average of 50%, and the level of protection increases with duration of use (10) (Fig. 24.4). Therefore, protection against ovarian cancer is probably the most important noncontraceptive benefit of OCPs. A meta-analysis reanalyzing data from 45 epidemiologic studies, which included 23,257 women with ovarian cancer and 87,303 controls, showed that in high-income countries, the use of OCP reduced ovarian cancer incidence from 1.2 to 0.8 per 100 users and mortality from 0.7 to 0.5 (15). These data confirmed not only that women who use OCP are at reduced risk of ovarian cancer, but also that the protection continues for decades after OCPs are discontinued. Furthermore, the reduction in risk is greater with more prolonged use. However, OCP had little effect on risk for mucinous tumors, which is consistent with a different biology of these tumors (16) and the current understanding that most are metastatic from the gastrointestinal tract.
Incidence of Stage and Grade by Age |
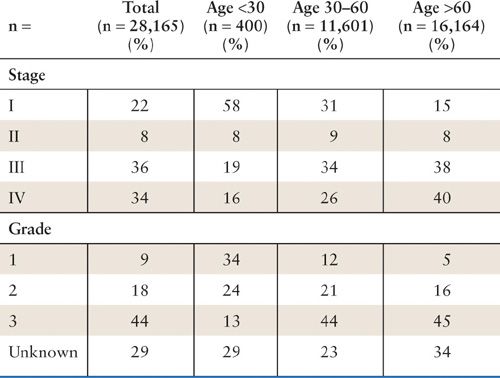
Source: Adapted from Chan JK, Urban R, Cheung MK, et al. Ovarian cancer in younger vs. older women: a population-based analysis. Br J Cancer. 2006;95(10):1314–1320, with permission.
The mechanisms underlying the profound and long-lasting protection against ovarian cancer provided by the OCPs are not well understood. The protective effects may be mediated by suppression of ovulation, reduction of gonadotropin levels, and/or induction of apoptosis (10). In view of the protective effects of parity and breast-feeding, however, the mechanism of protection might involve the reduced number of lifetime ovulatory cycles and its associated injury to the epithelial cells on the surface of the ovary. According to an older hypothesis (the “incessant ovulation” hypothesis) (17), ovarian cancer develops from an aberration in the repair process of the surface epithelium, which is ruptured and repaired during each ovulatory cycle. In support of this theory, it is well known that domestic egg-laying hens, which are forced to ovulate incessantly, have a high incidence of lesions believed to be ovarian-derived tumors with peritoneal carcinomatosis (18, 19). Alternative hypotheses addressing how OCPs may reduce ovarian cancer incidence center around their ability to treat endometriosis and reduce the risk of acquiring pelvic inflammatory disease (PID) (20), 2 conditions known to be associated with ovarian cancer (see below). However, these hypothesis are at odds with an origin of ovarian cancer in the fallopian tube (21; see Pathology section).
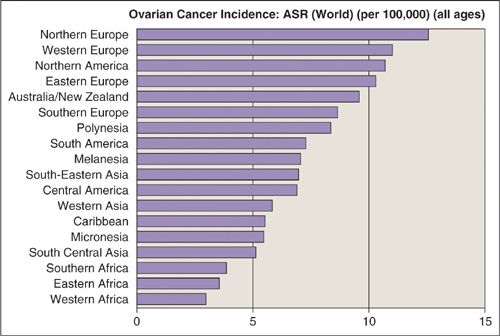
FIGURE 24.2. Age-standardized incidence rates (ASR) of ovarian cancer in the world.
Source: From Ferlay J, Bray F, Pisani P, et al. GLOBOCAN 2000: Cancer Incidence, Mortality and Prevalence Worldwide, Version 1.0. IARC Cancer Base No. 5. Lyon: IARC, 2001, with permission.
FIGURE 24.3. Summary of mortality from cancer according to body-mass index for U.S. Women in the Cancer Prevention Study II, 1982 to 1998. For each relative risk, the comparison was between women in the highest body-mass-index category (indicated in parentheses) and women in the reference category (body-mass-index, 18.5 to 24.9). Asterisks indicate relative risks for women who never smoked. Results of the linear test for trend were significant (p ≤ 0.05) for all cancer sites.
Printed with permission from: Calle EE, Rodriguez C, Walker-Thurmond K, et al. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638.
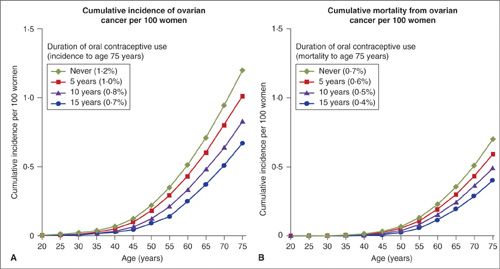
FIGURE 24.4. Absolute risk of ovarian cancer for women in high income countries, by duration of use of oral contraceptives. (A) Cumulative incidence of ovarian cancer per 100 women. (B) Cumulative mortality from ovarian cancer per 100 women.
Source: From Beral V, Doll R, Hermon C, et al. Ovarian cancer and oral contraceptives: Collaborative reanalysis of data from 45 epidemiological studies including 23257 women with ovarian cancer and 87303 controls. Lancet. 2008;371:303–314.
A case-control study has shown that progestin-only contraceptive users have a reduced risk (0.39) for developing ovarian cancer (22, 23). Moreover, the increase in progestin levels seen during pregnancy suggests that the protective effect of pregnancy may involve progestins as well as the reduction in the number of lifetime ovulations. Recent studies in primates indicate that the progestin component of OCPs has a chemopreventive effect by inducing apoptosis of ovarian surface cells that have undergone genetic damage (24). This is supported by the finding that women who use a long acting progestin-only contraceptives (Depo-Medroxyprogesterone Acetate—Depo Provera), which does not completely suppress ovulation, experience a protective effect similar to that observed with OCPs, which completely suppress ovulation (10).
Hormone Replacement Therapy
Currently, the primary indications for the prescription of HRT are severe postmenopausal symptoms and osteoporosis. Several prospective cohort studies (12, 25–28) examined how postmenopausal estrogen or estrogen/progestin HRT relates to the risk of developing ovarian cancer. Using data from the Breast Cancer Detection Demonstration Project, which is a cohort study of over 44,000 postmenopausal women, Lacey and colleagues found that use of estrogen-only HRT increased ovarian cancer RR by 1.6 (CI, 1.2–2), while the RR for women using an estrogen/progestin combination was not significantly increased (RR, 1.1; 0.64–1.7) (11). In a prospective, double-blind study, the Women’s Health Initiative (WHI), over 8,000 women who had not had a hysterectomy were randomized to either placebo or 0.625 mg conjugated equine estrogen with 2.5 mg medroxyprogesterone acetate (25). After an average of 5.6 years of follow-up, 20 women in the estrogen/progestin group were diagnosed with an invasive ovarian cancer versus 12 in the placebo group, which is not a significant difference (HR: 1.64; 0.78–3.45). There was no difference in the histologic subtype of ovarian cancers between the 2 groups. While the authors found the increase worrisome, they also pointed out that, because the absolute number of women who developed ovarian cancer was small, the trial had limited precision in this regard, and risk of ovarian cancer should therefore “not have an appreciable influence on most women’s decision making when seeking relief for moderate to severe vasomotor symptoms” (25). The observational “Million Women Study” (26), confirmed an increased risk with estrogen-only HRT (RR, 1.49; 1.2–1.81) and showed a lower risk with estrogen/progestin combination therapy (RR, 1.15; 1–1.33). This study also reviewed the cumulative incidence of gynecologic cancers, including ovarian, endometrial, and breast cancer in women taking HRT. The gynecologic cancer incidence per 1,000 women increased from 19 per 1000 in “never-users” to 26 per 1000 in current users of estrogen only, and to 35 per 1000 in current users of estrogen/progestin combinations (Fig. 24.5). The strength of this study, which followed nearly 1 million women, was that the results were adjusted for age at menopause, OCP use, BMI, smoking, and physical activity. HRT was found to be unrelated to the risk of mucinous ovarian cancer.
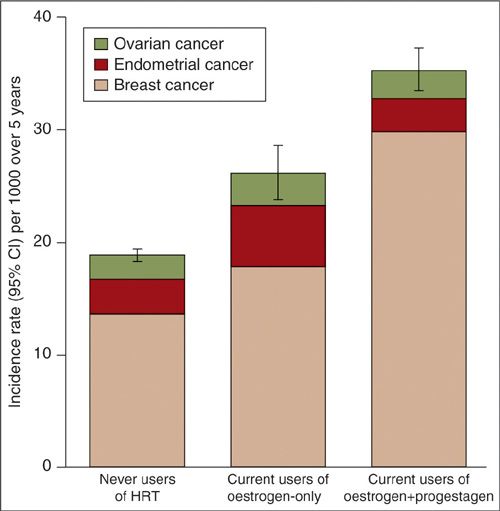
FIGURE 24.5. Standardized incidence rates (95% CI) for ovarian, endometrial and breast cancer per 1000 women in the study cohort over a 5-year period, for current users of various types of HRT and for never users*
*Incidence rates are standardized by age, region of residence, socioeconomic status, time since menopause, parity, use of oral contraceptives, BMI and alcohol consumption. Rates apply to women with a uterus and ovaries.
Source: Reprinted with permission from Beral V, Million Women Study Collaborators, Bull D, et al. Ovarian cancer and hormone replacement therapy in the Million Women Study. Lancet. 2007;369(9574):1703–1710.
In conclusion, a review of the major studies suggests a 30% to 58% increase in ovarian cancer risk in women who take HRT (12, 25–28). The risk is certainly increased in women taking estrogen-only HRT independent of whether they had a previous hysterectomy (11). Regular use of vaginal and transdermal estrogen also carries a slightly increased risk of ovarian cancer (26, 28). Most of the studies found that estrogen-progestin HRT and estrogen-only HRT are associated with essentially equal risk, calling a possible protective effect of progestins into question (12, 26, 28). However, one large study found no evidence that estrogen/progestin combinations increase the incidence of ovarian cancer (11). The risk of ovarian cancer was found to increase with duration of HRT exposure, and the largest risk was seen among women who used HRT for over 20 years. Most studies show that a treatment duration of less than 5 years carries no statistically increased ovarian cancer risk (26, 27), although one study did find that even a short duration of use increases risk (28). The negative impact of HRT on ovarian cancer risk is reversible after 2 years, when it approaches the risk observed in never-users (26, 28). This suggests that HRT has a direct growth-promoting effect on steroid receptors expressed on ovarian cancer cells (16,22). In contrast, the positive, risk-reducing effect of OCPs is maintained even 2 decades after the pill is discontinued.
The risk of excess ovarian cancer with HRT is relatively small, and could be represented as one extraovarian cancer for every 8,300 women who take HRT (28). This should not be the deciding factor in clinical decisions regarding HRT use. Current clinical practice is to prescribe HRT to patients with severe postmenopausal symptoms and to administer it at the lowest effective dose for a limited period of time (less than 5 years) (26, 27). Short-duration, low-dose use of HRT for menopausal symptom management seems to have an acceptable risk-benefit ratio for some women.
Surgery: Tubal Interruption and Hysterectomy
In general, studies have confirmed that both tubal interruption and hysterectomy at least partially protect against the development of ovarian cancer. The four most common surgical sterilization methods, tubal ligation, thermal injury, rings, and clips are probably equally effective in reducing risk. A large prospective cohort study, the Nurses Health study (29), confirmed smaller case-control studies and showed a RR of 0.33 (CI, 0.16–0.64) of developing ovarian cancer in women who had a tubal ligation compared to those who did not. The same study reported a weak inverse relationship between hysterectomy and ovarian cancer (RR, 0.67; CI, 0.45–1). The effect of hysterectomy was greater when the surgery was performed at an earlier age.
Since tubal ligation could serve as a form of secondary prevention of ovarian cancer for women at risk, a retrospective case-control study evaluated the effect of tubal ligation in patients with BRCA1 mutations (30). In this high-risk group, women who had undergone a tubal ligation had a considerably reduced risk of developing ovarian cancer when compared to women who had not (OR, 0.39; CI, 0.22 – 0.7). Women who both had a tubal ligation and had used OCP in the past had an even lower odds ratio of developing ovarian cancer (OR, 0.28; CI, 0.15–0.52). Possible explanations for the protective effect of tubal interruption against ovarian cancer include an impaired blood supply to the ovaries/distal tubes through the superior branch of the uterine artery leading in most women to earlier menopause (and fewer lifetime ovulations) and the possibility that occluding the tube blocks the upward flow of carcinogens from the uterus and reduces pelvic infection rates.
Inflammation: Pelvic Inflammatory Disease and Endometriosis
Pelvic Inflammatory Disease (PID) is a generalized infection of the female genital tract. Several small case-control studies had suggested that PID is associated with ovarian cancer. However, this association only gained wide acceptance with the publication of a large study (31) comparing the ovarian cancer incidence of 68,000 women who had experienced PID versus 136,000 who had not. In this well-designed study, the hazard ratio for ovarian cancer in patients with a history of PID (adjusted appropriately for confounding factors) was twice as high (HR 1.92) as that of controls, and was even higher (HR 2.46) in women who had at least 5 episodes of PID. Such indications of a dose-response effect always add credibility to epidemiologic findings (32).
Endometriosis, characterized by the ectopic growth of endometrial glands in the ovary and the abdominal cavity, affects 10% of women of reproductive age. In smaller case-control studies, a history of endometriosis has been consistently shown to be associated with clear cell and endometrioid ovarian carcinoma with an odds ratio of approximately 2. In many endometrioid and clear cell ovarian cancers, endometriosis is detected histologically adjacent to the carcinoma. Combining data from 13 ovarian cancer case-control studies (7,900 patients with ovarian cancer), the Ovarian Cancer Association Consortium (OCAC) published a definitive report that self-reported endometriosis increased the risk of clear cell (OR, 3.05; CI, 2.4–3.8) and endometrioid ovarian cancer (OR, 2.04; CI, 1.7–2.5) (33). In this report endometriosis was also associated, for the first time, with low-grade serous cancers (OR, 2.11; CI, 1.4–3.2), suggesting that these cancers, which are generally believed to arise from serous borderline tumors, can also arise from endometriotic implants.
There are 2 pathologic subtypes of endometriosis: displaced benign ectopic endometrial glands and atypical endometriosis. Given the high prevalence of endometriosis, it is likely that “benign” endometriosis acts through the creation of a microenvironment of chronic inflammation and is only one of several factors that can spur the development of clear cell and endometrioid cancers. In contrast, the endometriosis with cytological atypia and complex hyperplasia (“atypical endometriosis”) present in 2% to 3% of all patients who undergo surgery for endometriosis is most likely a direct precursor for type I/low-grade ovarian cancers (34,35). This hypothesis was supported by a recent study in which ARID1A gene mutations were detected in 30% of endometrioid and 46% of clear cell cancers, as well as in areas of atypical endometriosis that were adjacent to the cancers (36). Many genes expressed in endometriosis are also detected in endometrioid ovarian cancer, but do not overlap with genes expressed in high-grade serous cancers (37). In summary, endometriosis is associated with 3 type I (38) ovarian histiotypes: endometrioid, clear cell, and low-grade serous ovarian cancer (see also Pathology section).
A common mechanism linking endometriosis and PID to ovarian cancer risk may involve the intensive release of cytokines and infiltration of immune cells (macrophages) that accompany inflammation. Some (39,40), but not all (41) epidemiologic data suggest that the use of anti-inflammatory agents, including aspirin and nonsteroidal anti-inflammatory drugs, protects against ovarian cancer development.
Other Risk Factors: Diet, Smoking, Exercise
For the most part, the studies that have investigated whether diet affects ovarian cancer risk have had inconsistent and conflicting results. Any association between diet and cancer risk is likely difficult to decipher given the inaccuracy of food frequency questionnaires and seasonal differences in the supply of fresh food. Several studies have tried to determine whether vitamin A and β -carotene consumption affects ovarian cancer risk, but while some studies suggested a risk reduction, others could not confirm it.
In an Italian study, a diet favoring red meat was reported to confer an increased risk of ovarian cancer (42). However, the California Teachers Study, which had almost 100,000 participants, concluded that dietary factors are unlikely to play a major role in ovarian cancer development. In this study a RR reduction was only found with isoflavones, the phytoestrogens found in soy-based foods, some of which have antiestrogenic effects (RR, 0.6; 0.33–1) (43,44).
The Women’s Health Initiative Dietary Modification Randomized Controlled Trial evaluated prospectively the effects of reducing fat intake by at least 20% and increasing consumption of vegetables, fruits, and grains on ovarian cancer incidence in 48,835 postmenopausal women (45). The overall ovarian cancer HR was not statistically different between the 2 groups; however, the HR decreased with increasing duration of intervention: For the first 4 years, the risk for ovarian cancer was similar in the intervention and control groups; but then over the next 4 years, the risk was lower in the intervention group (0.38 cases per 1000 person-years in the intervention group versus 0.64 per 1,000 person-years in the comparison group [HR 0.6; CI, 0.38–0.96].
Indirect evidence for the effect of diet on the risk of ovarian cancer comes from the fact that women from geographic areas with a low incidence of ovarian cancer who relocate to a high-incidence region (North America, Europe) acquire the same risk as women who were born in that region, and by the increase of ovarian cancer incidence over time in Japan, which is transitioning to a more Westernized eating pattern (46).
Smoking is not generally considered a risk factor for ovarian cancer (47). However, current smoking seems to be associated with an increased risk of developing a mucinous ovarian cancer (OR, 1.78; 1.01–3.15) (48). Smoking cessation reduces this risk back to baseline over 20 years.
Currently, there is no convincing association between physical exercise and ovarian cancer risk and survival (49). An ongoing GOG trial is studying whether regular exercise, a low-fat diet, and high intake of vegetables after primary treatment for ovarian cancer affects recurrence (GOG #225).
In summary, factors that decrease the number of lifetime ovulations reduce the risk of developing ovarian cancer, while hereditary factors increase the risk. From a practical, clinical standpoint, only a positive family history will raise the suspicion of a predisposition to ovarian cancer in an asymptomatic woman.
HEREDITARY OVARIAN CANCER: BRCA AND HNPCC
Biology of BRCA-Associated Ovarian Cancer
The study of familial breast and ovarian cancer began in 1866 when the French physician Paul Broca noted a much larger than expected incidence of cancer in one family. Over 4 generations, 10 out of the 24 women in this family died from breast cancer, while several more individuals of both sexes developed other malignancies. Dr. Broca concluded that this excess of cancers could not reasonably be attributed to chance. Now that mutations in the high-penetrance BRCA1/2 genes and the MMR gene group associated with Lynch syndrome have been identified, we know that these hereditary mutations are the strongest known risk factors for the development of ovarian cancer. Approximately 15% of invasive epithelial ovarian cancers are estimated to be the result of autosomal dominant genetic factors with high disease penetrance, predominantly germline mutations in the BRCA1 or BRCA2 genes (65% to 85%), or in MMR genes (10% to 15%) (50).
The BRCA genes are inherited in an autosomal dominant fashion, which means that every first-degree relative of a mutation carrier has a 50% chance of carrying a mutation. The BRCA genes function as classic tumor suppressors, with loss of the function of both alleles required for cancer formation. Carriers are initially heterozygous for the BRCA gene mutation(s) in all cells and then the sporadic loss of the wild-type allele in epithelial breast or fallopian/ovarian cells results in a predisposition to cancer. The 2 BRCA proteins regulate cell cycle checkpoints and gene expression. Their most important function is probably participation in a specific DNA repair pathway, homologous recombination, which is used for the high-fidelity repair of double-strand DNA breaks. Because cells with BRCA1/2 mutations lack the ability to repair double strand breaks, they have increased genomic instability and a predisposition to malignant transformation. The ability of cells with BRCA mutations to repair DNA cross-links induced by platinum salts is impaired, which is hypothesized to explain the improved chemosensitivity and survival of patients with BRCA mutations (see below).
The prevalence of BRCA1 or BRCA2 mutations (over 1,000 have been identified) in the general population is about 1:300 to 1:800. However, specific ethnic populations founded by small ancestral groups, such as French Canadians, Icelanders, and Ashkenazi Jews, have a higher mutation rate arising from spontaneous “founder mutations.” For example, 2% to 3% of all Jewish women of Eastern European descent have one of 3 founder mutations (2 in BRCA1 187delAG and 5382insC; 1 in BRCA2 6174 delT). The cumulative lifetime risk of developing ovarian cancer for women with a BRCA1 mutation has been estimated at 39% to 54%, and for women with a BRCA2 mutation, 11% to 23%. The number of BRCA2-associated ovarian cancers is smaller overall. In comparison, the lifetime risk for ovarian cancer for women in the general population is 1.4% (51,52). As we understand more about the BRCA genes, the various BRCA1/2 genetic changes have been classified according to their functional effects. Class I mutations are present in 66% of BRCA1 mutation carriers and class II mutations in 25%, while class II mutations are rare in BRCA2 carriers (53) (see Table 24.2).
Also, loss of proteins in the homologous recombination pathway and loss of components for the Fanconi anemia pathway, will result in cells with defective homologous recombination that is comparable to cells with a BRCA mutation. This phenomenon is referred to as “BRCAness” (54). Therefore, while only 13%to 14% of women with ovarian cancer carry germline BRCA1/2 mutations, the TGCA analysis of almost 500 patients revealed that close to half of them had functional defects in one component of the homologous DNA repair pathway (55). Cancers with such functional defects have been hypothesized to exhibit similar clinical behavior as cancers associated with BRCA mutations, and means of identifying “BRCAness” of a subset of tumors that occur in women who do not carry a germline BRCA mutation are being sought. However, none of these classifiers have been clinically validated.
Clinical Features of BRCA-Associated Ovarian Cancer
In general, BRCA mutation carriers who develop ovarian cancer have a younger age at diagnosis, are more likely to have cancers of high-grade serous histology originating in the fallopian tube, are less likely to have borderline or mucinous tumors, and have a better prognosis than matched controls with sporadic ovarian cancer (50,56–58). A breast cancer precedes the ovarian cancer diagnosis in 37% of BRCA1-associated cases and 37% of BRCA2-associated cases (53).
Different Types of BRCA Mutations |
Mutation Type | Mutation Effect | Mechanisms |
Class I mutations | Loss of function mutations | – mRNA nonsense-mediated degradation – Instability of truncated protein – Deletion of regions regulating transcription → These changes result in reduced mRNA transcript, protein level or a truncated nonfunctional protein |
Class II mutations | Potentially stable mutant proteins: – dominant-negative functions – partially preserved normal function – loss of function | – missense substitutions – in-frame deletions and insertions – truncating mutations in the last exon of BRCA1/2 → these genetic changes might still allow for the expression of a partially functional protein and might have less detrimental effect |
Ovarian cancer cluster region mutations (OCCR | Mutations within nucleotide region c.2831–c.3847; c.6275–c.6401 | Mutations occurring in the central region are associated with a higher risk of ovarian and breast cancers compared to mutations outside this region |
In 1996, Rubin and colleagues reported results of a retrospective analysis suggesting that there are distinct clinical and pathologic features of BRCA1-associated ovarian cancer (56). Among 53 patients with germline BRCA1 mutations, the average age at diagnosis was only 48, and the vast majority of cancers were serous adenocarcinomas. Cancers associated with BRCA1 mutations had a relatively favorable prognosis, with an actuarial median survival of 77 months compared to 29 months for matched controls (Fig 24.6). Boyd et al. performed a retrospective cohort study of invasive ovarian cancers in patients of Jewish origin (67 BRCA1 and 21 BRCA2) (57). The average age at diagnosis was 54 for BRCA1 mutation carriers, 62 for BRCA2 mutation carriers, and 63 for Jewish women with sporadic ovarian cancers. The histology, grade, stage, and success of cytoreductive surgery were similar for hereditary and sporadic cases, although there were no mucinous tumors and only 2 clear cell tumors observed among 88 BRCA-associated cases, versus 5 mucinous and 7 clear cell tumors among 101 sporadic cases. The median disease-free interval after initial chemotherapy was 14 months for the BRCA-associated group and 7 months for the sporadic group (p < 0.001). While most series find that BRCA-associated cancers are most often of high-grade serous histology, one recent review suggested that BRCA1 and BRCA2 associated tumors are similar in histology and grade to sporadic cancers. A recent study of 1,119 BRCA1/2 associated ovarian cancers (53) reported 67% serous, 12% endometrioid, 2% clear cell and 1% mucinous cancers; the important clinical implication was that women with a nonserous carcinoma should still be considered for BRCA mutation testing (59). In a series of BRCA-associated ovarian cancers with centralized pathology review (60), cancers in BRCA carriers were compared to those in noncarriers. Among 220 women, mutation-associated tumors were of significantly higher grade and stage and less often mucinous, when compared to non-mutation-associated tumors. No mucinous and no borderline tumors were found in the mutation-associated group. Primary peritoneal carcinoma occurred rarely in both groups.
Extending the findings of improved survival of BRCA-associated ovarian cancers, a pooled analysis of 26 prospective, international studies published in 2012 found a 5-year overall survival of 36% for noncarriers, 44% for BRCA1, and 52% for BRCA2 mutation carriers. Even after adjusting for age, stage, grade, and histology, women with BRCA-mutations had a significantly longer survival, with BRCA2 mutation carriers having the best prognosis (58). These studies suggest that ovarian cancer developing in BRCA1/2 mutation carrier have specific clinical characteristics compared to sporadic ovarian cancer. It is probable that the improved survival of women with BRCA-associated ovarian cancers is related to the fact that loss of function of BRCA proteins, which participate in DNA damage repair, results in a more favorable response to platinum-based chemotherapy.
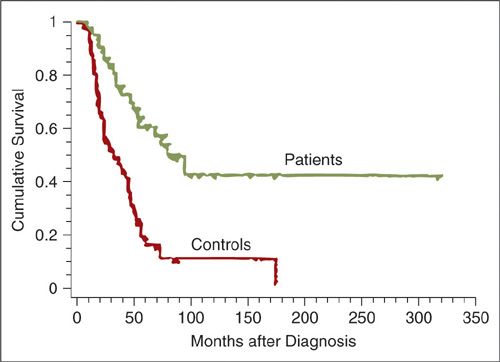
FIGURE 24.6. Actuarial survival among 43 patients with advanced ovarian cancer and BRCA1 mutations compared to matched controls without known mutations.
Source: Reprinted with Permission from Rubin SC, Benjamin I, Behbakht K, et al. Clinical and pathological features of ovarian cancer in women with germline mutations of BRCA1. N Engl J Med. 1996;335:1413–1416.
BRCA1/2 associated ovarian cancers are less likely to have platinum-resistant disease (14.9%) compared to sporadic ovarian cancer (31.7%), and when these patients recur, they tend to have a higher response to second-line platinum-based chemotherapy even in the setting of platinum-resistant disease (50). Platinum salts induce DNA crosslinks, which are recognized by DNA damage repair pathways, and are repaired by nucleotide excision repair and homologous recombination (61). It may be because BRCA1/2 associated high-grade serous cancers harbor defects in homologous recombination that platinum compounds are more efficient in these cancers (56,58). Indeed, a decrease in BRCA1 mRNA levels (PCR-based measurement) was associated with a significantly longer survival in 57 unselected ovarian cancer patients (62).
Deficiency in either BRCA1 or BRCA2 also causes profound cellular sensitivity to the inhibition of poly(ADP-ribose) polymerase (PARP) (63). The PARP enzyme is essential for the repair of DNA single-strand breaks. PARP inhibition blocks repair of single-strand breaks, and normal cells will compensate by using homologous recombination to bypass these lesions during DNA replication. Since BRCA-mutated cancer cells cannot perform DNA repair through homologous recombination, exposure to PARP inhibitors results in their death, while cells with intact homologous recombination pathways survive. This therapeutic mechanism is called “synthetic lethality,” and refers to the concept that 2 defective pathways that do not, individually, affect cell viability, will, in combination, be lethal (63,64). Several PARP inhibitors are in clinical development, and these drugs appear to have preferential single-agent activity in tumors of patients who carry germline BRCA mutations.
Genetic Testing/Counseling
BRCA1/2-directed genetic counseling is important because (a) it will help to identify women at risk of having a BRCA1/2 mutation, (b) it will allow for the implementation of preventive strategies in patients with known BRCA1/2 mutations and counseling of their families, and (c) it will guide therapy decisions in BRCA1/2-associated serous ovarian cancers. Given that a salpingo-oophorectomy significantly reduces the risk of developing ovarian and fallopian tube cancer, it is clinically meaningful to be aware if a woman carries a BRCA mutation. While the treatment (carboplatin/paclitaxel) for BRCA mutation-associated ovarian cancers is currently the same as treatment for sporadic ovarian cancers, the current development of PARP inhibitors may change this.
The following factors (59) are associated with a 20% to 25% chance of having an inherited disposition to breast and ovarian cancer and are recommended by the Society of Gynecologic Oncologists (SGO) for use in deciding which patients should be screened: Ashkenazi Jewish ancestry, young age at diagnosis, personal history of both breast and ovarian cancer, or a family history of ovarian cancer or breast cancer (particularly multiple first-degree relatives, or relatives with breast cancer at a young age, including paternal relatives), and a relative with a BRCA1/2 mutation. Women at risk for a BRCA mutation should be referred for genetic counseling, since they will have to make difficult decisions about genetic testing, and, especially if a mutation is found, ovarian and breast cancer screening and risk-reducing strategies including prophylactic surgery. Moreover, BRCA mutation carriers have an increased risk of pancreatic cancer (3% to 4%) and melanoma (5%). Considerable expertise and time is necessary to counsel these women well.
If a woman who is found to have a BRCA1/2 mutation chooses screening rather than prophylactic surgery, most practitioners will follow her with clinical pelvic exams, CA-125 testing, and pelvic ultrasound, but there is no evidence that these are effective screening strategies. Patients should also have regular breast examinations as well as annual mammograms and breast magnetic resonance imaging (MRI). Prophylactic bilateral mastectomy should also be discussed with BRCA mutation carriers, and patients should be informed of the techniques available for removing and reconstructing the breast, and the expected psychosocial and sexual effects. Counseling about risk-reducing salpingo-oophorectomy (RRSO) should balance the risks and symptoms associated with surgical menopause with the morbidity and high mortality of advanced serous ovarian cancer.
Most studies have shown that the reproductive and hormonal factors that affect ovarian cancer risk in the general population also affect risk for women who carry BRCA1 and BRCA2 mutations. Narod et al., from Toronto, initially reported a 60% reduction in ovarian cancer risk for BRCA1 or BRCA2 mutation carriers with OCP use for 6 or more years (65). However, while smaller studies have confirmed this observation, a large population-based study from Israel did not find a protective effect (66); therefore, the use of OCPs as a means to prevent ovarian cancer in BRCA mutation carriers prior to oophorectomy has remained controversial. Later, the Toronto group reported results from a greatly expanded database, which included 670 women with a history of BRCA1-associated ovarian cancer, 124 with a history of BRCA2-associated ovarian cancer, and 2,424 mutation carriers with no history of ovarian cancer (67). Use of OCPs reduced the risk of ovarian cancer in both women with BRCA1 mutations (OR, 0.56) and in those with BRCA2 mutations (OR, 0.39). Breast-feeding was also found to be protective for carriers of a BRCA1 mutation (OR, 0.74). An effect of similar magnitude was seen for breast-feeding in BRCA2 mutation carriers, but it was not statistically significant (OR,0.72). Although the Toronto group had previously reported that tubal ligation was protective against ovarian cancer in mutation carriers as well as noncarriers, the association in this expanded cohort was not significant for carriers of either a BRCA1 (OR,0.8) or a BRCA2 mutation (OR, 0.63). Pregnancy, which had previously been reported to be protective for women carrying a BRCA mutation) (66), was found to be protective for carriers of BRCA1 mutations (OR, 0.67), but was associated with increased risk for carriers of BRCA2 mutations (OR, 2.74). The reasons for this are not clear.
It is also important to offer genetic testing to patients with risk factors for a BRCA1/2 mutation who have already developed ovarian cancer. The identification of a BRCA mutation may impact their treatment, since it might indicate an increased sensitivity to platinum compounds in the first- and second-line setting (50) and PARP inhibitors. Also, their unaffected first-degree female relatives have a 50% probability of carrying the same mutation, and can be specifically tested for it. Those first-degree relatives who are found to be BRCA negative can be told that they are not at a statistically significant risk of developing ovarian cancer (68), while those who carry the mutation can be counseled on risk reducing strategies. The SGO criteria used to determine who should be screened for BRCA1/2 mutations are generally applied to women who have been diagnosed with ovarian cancer as well as to unaffected patients (see above [59]). However, by using these criteria for patients with the disease, practitioners will miss many women who have a BRCA mutation. A large study, which screened 1,342 unselected patients from the province of Ontario diagnosed with epithelial ovarian cancer, reported a mutation frequency of 13.4% (69). This study used multiplex ligation-dependent probe amplification, and, therefore, included the detection of large deletions that normally elude general sequencing. Women with ovarian cancer in their fourth life decade had the highest mutation rate (24%), as did women of Italian (43%), Jewish (30%), or Indo-Pakistani (29%) origin. Most importantly, 8% of the women in the study had no family history of ovarian cancer. Among Ashkenazi Jewish women with ovarian cancer, there is about a 29% to 40% chance that the disease is related to a BRCA1 or BRCA2 mutation (66,70). The Australian Ovarian Cancer group detected BRCA1/2 germline mutations in 22% of patients with high-grade serous cancers, 44% of whom did not have a family history of cancer (50).
Given these findings (50,54,55,69), the current complicated criteria for testing (59), advances in sequencing technology (55), and potential implications for selecting treatments (50,54), it has been suggested that testing for germline BRCA mutations should be routinely offered to all women with nonmucinous high-grade epithelial or serous ovarian cancer, regardless of family history. Such strategies are already employed in some Canadian provinces (Ontario, British Columbia) and are being considered in Australia.
Finally, it is important to be aware of legislation that address issues of discrimination and privacy that are raised by the prospect of increasingly comprehensive genetic information on each patient. The Genetic Information Nondiscrimination Act (“GINA”) (2008) prohibits health insurers and employers from discriminating on the basis of genetic information (71). Rules governing patient privacy and confidentiality prevent a physician from disclosing genetic test results to a relative. ASCO guidelines suggest that the ethical duty to warn a relative of genetic risk is satisfied if the doctor explains to the patient that a hereditary cancer syndrome has implications for other family members, advises the patient to share information with them, and offers genetic counseling for those family members who are interested and at risk.
Prophylactic Salpingo-Oophorectomy for Prevention of BRCA-Associated Ovarian Cancer
Indication for Surgery
There is, at this time, no scientific evidence that the current methods of screening can detect ovarian cancer early. The currently widely used screening procedures (clinical exam, CA-125, pelvic ultrasound) have such low specificity and sensitivity that their utility in detecting ovarian cancer at a curable stage is highly questionable (72). Such screening is even less efficient in young premenopausal women because ovulating women may have functional cysts or a hemorrhagic corpus luteum mistaken for a suspicious mass.
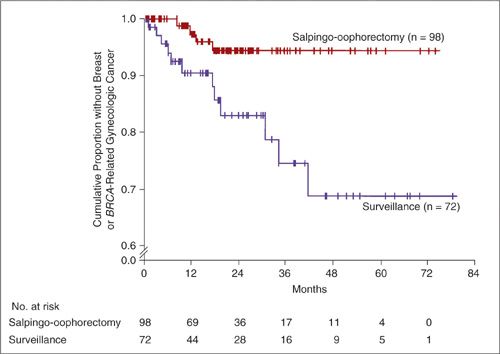
In contrast, the evidence does support the efficacy of RRSO for the early detection of ovarian cancer and its prevention in unaffected patients (73–76). In 2002, 2 large prospective series clearly demonstrated that RRSO reduced the risk of developing müllerian carcinoma (ovarian, fallopian tube, and peritoneal cancer) in patients with BRCA1/2 mutations (73,74). Kauff and colleagues from Memorial Sloan-Kettering prospectively studied 170 women with either BRCA1 or BRCA2 mutations for 6 years. Ninety-eight women who underwent RRSO were compared with 72 women who elected surveillance. As can be seen in Figure 24.7, the RRSO group had significantly fewer BRCA-related gynecologic and breast cancers than the surveillance group. Another prospective study, of a larger cohort of women, allowed us to understand the effects of RRSO in BRCA1 and BRCA2 patients separately. In this multicenter study of 1,079 patients, RRSO reduced ovarian cancer risk in women with BRCA1 mutations by 85% and reduced breast cancer risk in women with BRCA2 mutations by 72%. There was also a 39% reduction in breast cancer risk in women with BRCA1 mutations, and a reduction in gynecologic cancers in women with BRCA2 mutations but these were not statistically significant. The absence of a significant reduction in ovarian cancer in BRCA2 mutation carriers was probably attributable to the fact that most women with BRCA2-associated ovarian cancer are over 60 years old, while the median age of the women in the study was 46 years (69,75). In another large study coordinated by the Toronto group, 1,828 known carriers of a BRCA1 or BRCA2 mutation were identified from an international registry of 32 centers. The overall reduction in risk of müllerian cancers with RRSO was 80%; the estimated cumulative incidence of peritoneal cancer at 20 years after oophorectomy was 4.3%, with most cases occurring less than 5 years after RRSO (76).
FIGURE 24.7. Kaplan–Meier Estimates of the Time to Breast Cancer or BRCA-Related Gynecologic Cancer among Women Electing Risk-Reducing Salpingo-oophorectomy and Women Electing Surveillance for Ovarian Cancer..
P = 0.006 by the log-rank test for the comparison between the actuarial mean times to cancer. A Cox proportional-hazards model for multiple end points, which took into account the different proportions of women in the 2 groups who had breast tissue at risk, yielded a hazard ratio for subsequent breast cancer or BRCA-related gynecologic cancer after risk-reducing salpingo-oophorectomy of 0.25 (95% confidence interval, 0.08 to 0.74).
Source: From Kauff ND, Stagopan JM, Robson ME, et al. Risk-reducing salping-oophorectomy in women with a BRCA1 or BRCA2 mutation. N Engl J Med. 2002;346:1609–1615, with permission.
Given the strong evidence that RRSO is protective against the development of ovarian cancer, The National Comprehensive Cancer Network (NCCN), the American College of Obstetrics and Gynecology (ACOG) and the Society of Gynecologic Oncology (SGO) (59) have recommended that prophylactic oophorectomy be considered in women with ovarian cancer syndromes at age 40 years or after childbearing is completed. Because BRCA2 mutation carriers will develop ovarian cancer at an average age of 58, and only 2% to 3% of these women will develop ovarian cancer by age 50 as compared to 10% to 21% of women with BRCA1 mutations, delaying surgery in BRCA2 mutation carriers could be considered (69). However, women with BRCA2 mutations have a 26% to 34% risk of developing breast cancer by the age of 50, and the evidence suggests that the breast-cancer risk reduction conferred by RRSO is greater when the ovaries are removed earlier (77).
Surgery
RRSO substantially decreases ovarian cancer risk. Some risk of primary peritoneal cancer remains after RRSO although that risk may largely arise from an ovarian remnant, an incompletely removed fallopian tube, or a microscopically metastasized, occult cancer that could not be recognized at the time of surgery.
The informed consent discussion for RRSO surgery should include not only information about the general risks of surgery, but also information about the likely side effects of bilateral salpingo-oophorectomy. Permission to perform a full staging or debulking procedure if cancer is found should also be obtained. The rate of occult cancer detected with RRSO (which requires an additional surgical procedure) can be up to 10% in a tertiary referral center (21,78) though the rate drops to approximately 3% when centers that do not perform an extensive pathologic review of the fallopian tube are included (79). Hysterectomies are not performed routinely, because there are no reports indicating that the intrauterine portion of the fallopian tube gives rise to a fallopian tube cancer. However, hysterectomy may be indicated if there is other uterine pathology, if the uterus contributes to incontinence or bleeding disorders, to reduce endometrial cancer risk for patients with Lynch syndrome who are also at risk for endometrial cancer (see below), or to simplify HRT.
It is usually possible to perform a RRSO laparoscopically as an outpatient procedure. Occasionally a laparotomy will be necessary due to extensive intra-abdominal/pelvic adhesions. After a thorough surveillance of the entire abdominal cavity including the upper abdomen, peritoneal washings are performed and abnormal areas biopsied. The ureter is visualized and the infundibulopelvic vessels are transected about 2 cm superior to the ovary to assure that the entire ovary has been removed. The tube and the superior branch of the uterine artery are transected very close to the uterine cornu. A frozen section is only prepared if there is a gross abnormality of the ovary or any other suspicious tumor. Random biopsies of the omentum and peritoneum have not been found to lead to improved detection of occult cancers (78). Many patients presenting for RRSO will have had a previous reconstruction of their breast using some variation of a rectus abdominus myocutaneous flap. Since these procedures can lead to umbilical translocation in relation to the aortic bifurcation, higher camera port placement may be required (80). In general, RRSO is associated with a very low risk of operative complications. In a Memorial Sloan-Kettering study, four out of 80 RRSOs performed laparoscopically had complications caused by adhesions and trocar injuries, which are known complications associated with operative laparoscopy (74).
Because high-grade serous “ovarian cancer” is now believed to arise in the fallopian tube, the option of removing the fallopian tube and leaving the ovary in situ is being debated. As of this time, this approach is not supported by published data, and we believe that it remains strictly investigational.
Postoperative Results
Women who have chosen to undergo RRSO generally report a good overall quality of life. The surgery is often accompanied by a significant decrease in perceived risk, and therefore a decrease in anxiety (81). Acute surgical menopause, however, can have a significant negative effect on quality of life (82). Surgical menopause affects bone health and can cause a decrease in sexual desire, vaginal atrophy and dyspareunia, affecting sexual functioning and leading to decrease in sexual desire, discomfort, and avoidance of intimacy. Many patients suffer from vasomotor symptoms, such as hot flashes and night sweats, which lead to sleep disturbances. These symptoms can be alleviated but not completely eliminated by HRT (81). It has been reported that short term HRT (approximately 3 years duration) does not negate the protective effect of RRSO on the development of subsequent breast cancer (83). This was confirmed by Eisen et al., who showed that HRT after RRSO is not associated with an increase in breast cancer risk in BRCA mutation carriers (84). Still, decision-making regarding menopausal therapies in women with BRCA mutations who are at increased risk of breast cancer is challenging because of the theoretical risk that HRT will promote growth of occult breast tumors. Alternative treatments for vasomotor symptoms, such as venlafaxine and gabapentin, should be discussed in counseling.
The Pathologic Examination of Risk-Reducing Salpingo-Oophorectomy Specimens
A family history of ovarian cancer and/or BRCA mutation status should be shared with the pathologist, since in patients with benign gynecologic disease only one slide from the fallopian tube and ovary is normally reviewed (21,78). In the setting of a known history of genetic predisposition to breast and ovarian cancer, most pathologists will submit the entirety of the fallopian tubes and ovaries for microscopic examination. The SEE-FIM (sectioning and extensively examining the fimbriated ends) protocol is widely used. Basically, this involves serially sectioning the tube meticulously, stopping before the fimbriae. The fimbria is amputated and sectioned longitudinally, thereby maximizing exposure. Deeper sections need to be obtained if foci of atypia are to be identified histologically. Foci of in situ or invasive occult carcinoma may be very subtle, and are often less than 1 mm in maximum diameter. (See also Pathology section.)
Microscopic occult carcinomas have been identified in RRSO specimens in about 2% to 9% of BRCA mutation carriers, generally involving the tubal fimbriae. In one prospective series, 7 ovarian, and 3 tubal carcinomas (and 1 case in which washings showed malignant cells but no primary cancer was identified) were found among 490 women who underwent RRSO (76). Powell and colleagues report that in 111 consecutive BRCA-positive patients treated at a single institution, 9% had occult neoplasia (78). Suspicious epithelial cells, clearly distinct from mesothelial cells, are occasionally identified in cytology specimens. Colgan et al. found malignant cells in 3 of 35 pelvic washings. One microscopic ovarian surface carcinoma and 1 in situ tubal carcinoma were found; no carcinoma could be identified in the third patient. Twenty-two percent of specimens showed endosalpingiosis (85). Positive cytology specimens only rarely lead to the discovery of early-stage tubal carcinomas (21,78) although sometimes, as mentioned, malignant cells are present in washings at RRSO and there is no identifiable carcinoma by histology (76).
Lynch Syndrome—Hereditary Nonpolyposis Colorectal Cancer Syndrome
Epithelial ovarian cancer is also a component of Lynch syndrome II (Hereditary nonpolyposis colorectal cancer syndrome—HNPCC). In addition to a predisposition to develop colorectal and endometrial cancer, woman with this syndrome have a 10% to 13% lifetime risk for developing ovarian cancer (86). Women at risk for Lynch syndrome are identified using either the Amsterdam II or the revised Bethesda criteria, attempting to enrich for patients who are likely to have a hereditary origin of their cancer. However, even though the Bethesda criteria have been revised to improve identification, they may still miss as many as 28% of patients with Lynch syndrome.
Lynch syndrome-related tumors exhibit a lengthening or shortening of DNA repeat sequences, which leads to microsatellite instability (MSI), caused by an inability to repair DNA replication errors. This syndrome is a result of germline mutations in genes involved in the DNA mismatch repair pathway, such as MSH2 and MLH1 which account for about 90% of the mutations detected in families with Lynch syndrome, with MSH2 being particularly associated with an excess of endometrial and ovarian carcinomas. Other genes in the MMR family, including MSH6, PMS1, and PMS2, account for 10% of HNPCC-related cancers (86). To identify patients with HNPCC 2 approaches are used: For MSI analysis, DNA is extracted from macrodissected tumors using paraffin sections, and short tandem repeats are amplified. Immunohistochemical stains are performed for MSH2, MSH6, MLH1, and PMS2. If a MMR protein is absent, despite appropriate controls, confirmatory sequencing is performed. MSI can be due to epigenetic changes as well as to Lynch syndrome. For example, MLH1 promoter hypermethylation or BRAF gene mutations can result in MSI.
A recently published French multicenter study reviewed the cancer incidence in 537 families with Lynch syndrome (87). For women in the study, the cumulative risk for Lynch syndrome-associated cancers was 19% by age 50 and 54% by age 70. The age specific cumulative risk for ovarian cancer by age 70 was 20% for MLH1 mutation carriers, 24% for MSH2 mutation carriers, and 1% for those with MSH6 mutations (Fig. 24.8). This study clearly showed that MSH6 mutation carriers have much lower cancer risks than MLH1 and MSH2 mutation. These findings raise the question of whether women with a MSH6 really need prophylactic surgery, especially if no other family member has been affected by cancer. However, a smaller cohort study did not confirm this low ovarian cancer risk in women affected by MSH6 mutations and found that a third of cases of ovarian cancer identified among MMR mutation carriers were affected by a MSH2
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree