THYROID
Key Points
• Evaluation of thyroid function should be performed in patients with a history of thyroid disorder, medical conditions commonly associated with thyroid disease, strong family history, or suggestive symptoms.
• The recommended iodine intake in pregnancy is increased to 250 μg/d to compensate for increased renal iodine clearance and fetal and placental utilization (1,2).
• Thyroid hormone is critical for normal central nervous system development during embryogenesis. Women with normal thyroid function increase the production of thyroid hormone by 50% in the first trimester.
• Until method- and trimester-specific free T4 levels are established, thyroid-stimulating hormone (TSH) is the most reliable means to assess thyroid function in pregnancy (1).
Background
Pregnancy Physiology
• TSH from the anterior pituitary stimulates thyroid production of thyroxine (T4) and levothyronine (T3 ), the important metabolic mediators and essential hormones for normal fetal and neonatal brain development. Iodine is required for T4 and T3 synthesis.
• Human chorionic gonadotropin (hCG) has direct thyroid-stimulating activity and transiently increases T4 production in the first trimester.
• TSH levels decrease in the first trimester via negative feedback as hCG peaks (3), and asymptomatic, mildly decreased TSH does not indicate hyperthyroidism. The median TSH during the first trimester is 0.8 mIU/mL (0.03 to 2.3 mIU/mL). TSH rises back to the normal range in the second and third trimesters.
• Levels of total thyroxine (TT4) and triiodothyronine (TT3) increase during pregnancy and remain 1.5 times above the normal nonpregnant reference range throughout pregnancy.
• Thyroid-binding globulin (TBG) is the primary binding protein for T4 and T3 in plasma. Estrogen stimulation during pregnancy increases serum TBG production by 50%. Free T4 (FT4) and free T3 (FT3) are more accurate measures of thyroid status but must be interpreted carefully as trimester-specific normative ranges have not been established and results from common clinical assays vary (3).
• The transfer of maternal thyroid hormones across the placenta to the fetus varies, but thyroid autoantibodies, antithyroid medications, and iodine can passively cross the placenta and affect the fetus.
• The placenta is relatively impermeable to TSH. A although uptake of T4 and T3 from the maternal circulation to the placenta is rapid, transfer to the fetus is regulated via placental deiodinases and thyroid hormone transporter proteins (4,5).
• Thyroid function in the newborn changes rapidly in the first 48 hours of life.
• Fetal secretion of thyroid hormone is significant after the first trimester, and at the time of delivery, 20% to 30% of fetal thyroid hormones are of maternal origin.
• Immediately after delivery, a significant fall in the newborn’s temperature causes a rapid increase in TSH and thyroid hormone secretion. Following this burst of thyroid hormone, TSH declines exponentially within 48 hours due to negative feedback. In term infants, T4 and T3 remain elevated for approximately 6 weeks (6).
• Delayed treatment of congenital hypothyroidism increases the risk for mental retardation and other neurologic sequelae. Newborn screening for hypothyroidism on days 2 to 4 of life is mandatory to prevent lifelong impairment (6).
HYPERTHYROIDISM
Key Points
• The prevalence of hyperthyroidism in pregnancy is 0.2%.
• Graves disease is the most common cause of hyperthyroidism in pregnancy.
• Standard treatment of Graves disease in pregnancy is with antithyroid drugs (ATD), using the lowest dose that reduces FT4 to the upper end of the normal range.
Background
Definition
• Hyperthyroidism is defined by the suppression of serum TSH below the lower limits of the trimester-specific range and elevated serum FT4 and/or FT3 above the nonpregnant reference range.
• Subclinical hyperthyroidism is defined as a suppressed TSH concentration and serum FT4 and/or FT3 within the nonpregnant reference range.
Differential Diagnosis and Etiology
• Gestational transient thyrotoxicosis is characterized by elevated FT4 and suppressed TSH levels in the first trimester, with the absence of a goiter, history of hyperthyroidism, or TSH receptor antibodies (TRAB). It is caused by the thyrotropic effects of hCG early in pregnancy and spontaneously improves with the decline of hCG in the second trimester. Gestational thyrotoxicosis is commonly associated with hyperemesis gravidarum, multiple pregnancies, and gestational trophoblastic diseases, but has not been correlated with poor pregnancy outcomes (7).
• Hyperemesis gravidarum is a clinical diagnosis of persistent vomiting, evidence of starvation (i.e., ketonuria) and 5% weight loss. It complicates 0.5% to 2% of pregnancies, and up to 60% to 70% of women will have suppressed TSH and elevated FT4 levels.
• Trophoblastic hyperthyroidism is seen in up to 64% of women with placental tumors. The hyperthyroidism may be severe due to the thyrotropic activity of partially desialylated hCG. It is commonly treated by removal of the hydatidiform mole or chemotherapy-directed treatment for choriocarcinoma (7).
• Graves disease is an autoimmune disorder caused by the production of antibodies that bind to the TSH receptor and accounts for 85% to 95% of hyperthyroidism in pregnancy. TRAB can be measured in serum and have either thyroid-stimulating or thyroid-blocking effects. Measurement of thyroid-stimulating immunoglobulins (TSI) is a bioassay that distinguishes between these effects with stimulating activity being the early universal feature of Graves’. TSI cause general thyroid activation with increased growth, vascularity, and excess secretion of thyroid hormones independent of TSH. This is generally associated with signs and symptoms of thyrotoxicosis.
• The least common causes of thyrotoxicosis in pregnancy are toxic nodular goiter, functional adenomas, thyroiditis, or excessive intake of thyroid hormones.
Diagnosis and Evaluation
• The key features of Graves disease are abnormal thyroid function tests, a goiter, positive TRAB, and less commonly findings of Graves ophthalmopathy or dermopathy.
• Palpable thyroid masses or nodules should be evaluated with thyroid ultrasound and fine needle aspiration is recommended for nodules suspicious for malignancy (8). Radioactive iodine scans, used to visualize the functional anatomy of the thyroid in nonpregnant women, are contraindicated during pregnancy.
History and Physical Exam
• Thyroid hormones affect metabolism in virtually all tissues. Common clinical symptoms of hyperthyroidism include tachycardia, palpitations, weight loss, nervousness, muscle weakness, heat intolerance, hair loss, or excessive sweating. Findings specific for Graves disease are infiltrative dermopathy (pretibial myxedema) and exophthalmos, due to inflammation, edema, and hyaluronate accumulation in the extraocular muscles.
Diagnostic Testing
• Serum TSH measurement is the mainstay for the diagnosis of hyperthyroidism. If the TSH is low, FT4 evaluation is recommended. Serum FT3 assessment is advised if the FT4 is within normal range.
• Circulating TRAB are present in greater than 90% of patients with active Graves disease. Antibodies fluctuate throughout gestation and decrease through the second and third trimesters (7). Commonly, values will increase in the postpartum period when patients may experience an exacerbation of Graves disease.
Pregnancy Complications
• Treatment is essential. Uncontrolled hyperthyroidism increases the risk for spontaneous abortion, preterm birth, preeclampsia, intrauterine growth restriction, low birth weight, and fetal demise (9,10).
• Thyroid storm is an accelerated, extreme presentation of hyperthyroidism that occurs in 1% of pregnancies and is a medical emergency. Patients present with usual signs and symptoms of hyperthyroidism in addition to fever, sinus tachycardia and/or atrial arrhythmia, heart failure, hyperbilirubinemia, and mental status changes (10).
• Fetal hyperthyroidism complicates 1% to 5% of pregnancies with Graves disease. Transfer of maternal antibodies to the newborn and the capacity for the neonatal thyroid to respond to TSI cause this condition. Findings suggesting fetal hyperthyroidism are a goiter on ultrasound, fetal heart rate greater than 160 bpm, evidence of heart failure, arrhythmias, advanced bone age, growth restriction, craniosynostosis, and hydrops (9).
• Fetal hypothyroidism is a risk of overtreatment with ATD or passive transfer of blocking thyroid antibodies. Cordocentesis, fetal blood sampling, should only be performed for cases in which definitive fetal thyroid function is uncertain from ultrasound assessment and the results would impact management (8,11).
Treatment
• Iodine-131 exposure is contraindicated in pregnancy for the risk of fetal thyroid ablation, and pregnancy should be avoided for at least 4 to 6 months after exposure (8,10).
• Women with Graves disease should have TRAB titers assessed at initial prenatal visit and between 22 and 26 weeks to assess the risk of neonatal hyperthyroidism. Measurement of TSI in the latter phase of the third trimester is predictive of neonatal hyperthyroidism.
• Medical management: Propylthiouracil (PTU) and methimazole (MMI) are antithyroid medications that inhibit thyroid hormone synthesis. PTU also decreases peripheral conversion of T4 to T3 and is the recommended thioamide drug in the first trimester. Embryopathy, primarily aplasia cutis, and choanal or esophageal atresia are rare complications of MMI and almost never seen with PTU. With the infrequent but potentially fatal hepatotoxicity associated with PTU, therapy should be switched to MMI after the first trimester unless the patient is intolerant of MMI (2,8).
• Dosing: Initial treatment with PTU is 50 to 100 mg every 6 to 8 hours or MMI 10 to 20 mg every 12 hours (10). As symptoms improve and FT4 decreases, the dosing of ATD is reduced. In stable patients, PTU is usually given two to three times daily and MMI once daily. The goal of therapy is to maintain FT4 at or just above the upper limit of the nonpregnant reference range with the lowest ATD dose (8,10).
• FT4 and TSH should be measured every 2 to 4 weeks after initiating ATD, and every 4 to 6 weeks after the FT4 goal is reached to guide dose adjustments. Serum TSH may remain suppressed even after FT4 responds and should not be used alone to guide treatment.
• Minor drug side effects include rash, urticaria, arthralgias, nausea, metallic taste, and pruritus, which often resolve spontaneously without significant sequelae.
• Agranulocytosis may occur in 0.5% of treated individuals, presenting with fever, sore throat, and neutropenia. Immediate discontinuation of thioamide drugs with the development of a sore throat and fever is recommended until a white blood cell count and differential has been performed (10).
• Although small amounts of PTU and MMI are found in breast milk, breast-feeding is considered safe in mothers taking these medications.
• Thyroidectomy is indicated if ATD are ineffective, adverse reactions occur, or with a symptomatic goiter. Surgery is optimally performed in the second trimester.
HYPOTHYROIDISM
Key Points
• Hypothyroidism has a prevalence of 0.3% to 1.5% in the United States and is 5 to 10 times more common in women than men (2).
• Iodine deficiency is the most common cause of hypothyroidism worldwide. In developed countries, autoimmunity is the most common etiology. Hashimoto disease (chronic lymphocytic thyroiditis) and atrophic thyroiditis are the two manifestations of autoimmune thyroid disease leading to hypothyroidism.
• Women are advised to reach an euthyroid state with serum TSH in the lower limit of normal prior to conception for optimal pregnancy outcomes (12,13).
• Pregnant women with preexisting hypothyroidism invariably require an increase in thyroxine dose by 30% in the first trimester.
Background
Definition
• Hypothyroidism is defined as a serum TSH elevated above the trimester-specific range and a FT4 below the nonpregnant reference range.
• In subclinical hypothyroidism, there is elevated serum TSH with normal FT4 levels.
Differential Diagnosis and Etiology
• Hypothyroid symptoms are nonspecific. Anemia, depression, and pregnancy can mimic many of the symptoms experienced.
• Hashimoto disease is the most common cause of primary hypothyroidism. It is characterized by a small firm goiter due to lymphocytic infiltration, painless inflammation, and destruction of thyroid cells.
• Prior ablative radioiodine therapy or thyroidectomy.
• Medications altering thyroid hormone (see Table 18-1).
• Endemic iodine deficiency.
• Secondary hypothyroidism from pituitary disease and TSH deficiency such as Sheehan syndrome, lymphocytic hypophysitis (LH), or history of hypophysectomy.
• Tertiary hypothyroidism due to hypothalamic disease is rare. Sarcoidosis and histiocytosis are the most common causes of tertiary hypothyroidism (10).
Table 18-1 Drugs that Affect Thyroid Function
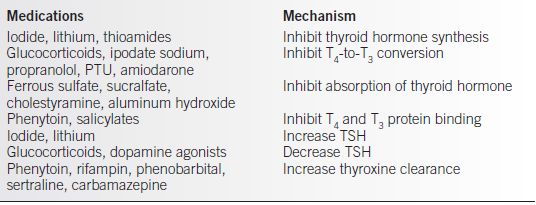
Diagnosis and Evaluation
History and Physical Exam
• Diagnosis in pregnancy is difficult because many of the symptoms associated with hypothyroidism are also common in pregnancy.
• Common clinical signs and symptoms of hypothyroidism may include the following: fatigue, lethargy, dry skin, weight gain, cold intolerance, impaired memory, edema, or depression. Goiter is common with iodine deficiency or Hashimoto thyroiditis.
Diagnostic Testing
• Measurement of serum TSH is the mainstay for the diagnosis of hypothyroidism. If the TSH is abnormal, antithyroid antibodies and FT4 are recommended.
• Elevated anti-thyroid peroxidase antibodies (anti-TPO Ab) and antithyroglobulin anti-bodies (anti-Tg Ab) are consistent with thyroid autoimmunity and are associated with higher rates of miscarriage and preterm labor.
Pregnancy Complications
• Hypothyroidism is associated with an increased rate of spontaneous abortion, especially in women with positive antithyroid antibodies (8). Thyroid dysfunction increases the risks for pregnancy complications such as preeclampsia, placental abruption, prematurity, premature rupture of membranes, postpartum hemorrhage, intrauterine demise, and low birth weight (10,14).
• Maternal hypothyroidism is associated with impaired neuropsychological development in the child (15).
Treatment
Medications
• Synthetic levothyroxine (T4) is the treatment of choice for hypothyroidism (16,17).
• If hypothyroidism is newly diagnosed in pregnancy, levothyroxine should be started at 1.5 to 2 μg/kg. Patients with malabsorption, complete thyroidectomy, or prior ablative radioiodine may require higher concentrations. Thyroxine is best taken on an empty stomach at bedtime or first thing in the morning. Calcium, antacids, sucralfate, and caffeine can interfere with absorption.
• Higher doses of levothyroxine are required in 85% to 90% of patients with prior hypothyroidism early in the first trimester (13,18). In these patients, increasing levothyroxine 30% (~2 additional tablets per week) once pregnancy is established decreases the risk of hypothyroidism in the first trimester. Patients should strive for an euthyroid state prior to conception and immediately seek care to have thyroid function studies performed when pregnancy is confirmed for optimal management (12,13,18).
• TSH should be measured 4 to 6 weeks after each dose adjustment and each trimester once the optimal serum TSH levels have been reached. During pregnancy, TSH goals are less than 2.5 mIU/L in the first trimester and 3.0 mIU/L in the second and third trimesters.
• TSH 4 to 10Increase dose by 25 to 50 μg daily
• TSH 10 to 20Increase dose by 50 to 75 μg daily
• TSH greater than 20Increase dose by 75 to 100 μg daily
• Reduce levothyroxine to prepregnancy dose after delivery and recheck TSH 6 weeks later.
POSTPARTUM THYROID DYSFUNCTION
Key Points
• The prevalence of postpartum thyroid dysfunction (PPTD) is 5% to 10% (10,17,19).
• Women with autoimmune disorders such as type 1 diabetes mellitus, Addison disease, and systemic lupus erythematosus are at a higher risk for PPTD, a form of autoimmune thyroid disease (2,17,19,20).
• After one episode, patients have a 70% risk of recurrence in a subsequent pregnancy. The risk of permanent hypothyroidism is 20% to 64% and can occur as soon as the first year postpartum (17,19). Women with PPTD should have annual serum TSH screening.
Background
Definition
• PPTD is a transient autoimmune thyroiditis during the 1st year postpartum in women who were euthyroid prior to pregnancy.
• The typical presentation is with a period of transient thyrotoxicosis, followed by transient hypothyroidism, and return to an euthyroid state by the end of the first year postpartum. Patients may present with transient isolated thyrotoxicosis or isolated hypothyroidism followed by an euthyroid recovery period.
Differential Diagnosis and Etiology
• Up to 50% of women with positive TPO-Ab during pregnancy develop PPTD. Thyroid gland inflammation due to lymphocytic thyroiditis is thought to result from the immunologic rebound following selective immunosuppression of normal pregnancy (19).
• Anemia and depression may cause symptoms similar to PPTD.
DIAGNOSIS AND EVALUATION
History and Physical
• Thirty percent of patients with PPTD are asymptomatic. In patients with underlying Hashimoto disease, a small firm goiter is palpable.
• Thyrotoxic symptoms are less pronounced than in Graves’ disease, and elevations of FT4 are lower. Frequent symptoms include fatigue, palpitations, nervousness, irritability, weight loss, and heat intolerance.
• Hypothyroid symptoms are often more significant and include fatigue, impaired memory and concentration, constipation, dry skin, cold intolerance, and myalgia/arthralgia.
Diagnostic Testing
• Typical laboratory parameters for hyper- and hypothyroidism are used for PPTD. Antithyroid antibodies are commonly associated with this disease.
• In contrast to Graves’ disease, PPTD is distinguished by a substantially reduced uptake of radioactive iodine. Radioactive iodine is contraindicated in lactating mothers.
Treatment
• Antithyroid medications are not effective for treating hyperthyroidism of PPTD.
• Propranolol may be used to mitigate some of the symptoms of hyperthyroidism such as tremor, heat intolerance, and palpitations, providing relief during the hyperthyroid phase. Patients should be educated to get adequate rest and hydration and avoid stimulants and symptom triggers.
• Hypothyroidism is treated with levothyroxine when the patient is symptomatic, planning or attempting pregnancy, currently pregnant, breast-feeding, or when serum TSH is abnormally elevated for greater than 6 months (17,19). The duration of levothyroxine therapy has not been firmly established, but patients may be weaned after 6 to 12 months of therapy to determine if the hypothyroid phase is permanent (2,10,17).
PARATHYROID DISORDERS
Key Points
• Primary hyperparathyroidism is a common endocrine disease with a prevalence of 2–3:1000 American women. However, hypoparathyroidism is less common in women of reproductive age, and the epidemiology in this group is not well defined (21,24).
• In pregnant women, hypercalcemia can be underestimated because of physiologic hypoalbuminemia, growing fetal calcium demands, and gestational hypercalciuria.
Background
Physiology in Pregnancy and Lactation
• Calcium regulation is critical for normal cell function and signaling. Pregnancy and lactation significantly alter calcium homeostasis. The parathyroid glands release parathyroid hormone (PTH), a peptide hormone that maintains calcium balance within a narrow range (21). PTH increases conversion of 25-OH vitamin D to the active 1, 25-OH vitamin D, increasing intestinal calcium absorption. PTH decreases renal clearance of calcium and increases osteoclast activity, causing calcium resorption from bone.
• During pregnancy, a total of 25 to 30 g of calcium is transferred to the fetus. The maternal intestinal calcium absorption doubles by the end of the first trimester, which is mediated by a twofold increase in 1, 25-dihydroxy vitamin D (1,25(OH)2 D3 ), the active form of vitamin D. Placental and maternal renal 1,25(OH)2 D3 synthesis is mediated by estrogen, human placental lactogen, prolactin, and parathyroid hormone–related peptide (PTHrP) as well as PTH (21).
• In pregnancy, total calcium concentrations in the blood decrease due to physiologic hypoalbuminemia (22). Maternal ionized calcium remains unchanged throughout pregnancy. PTH may be slightly suppressed or within the normal range. PTHrP is produced by the fetal parathyroid glands and the placenta, regulating active placental calcium transport to the fetus (21,22). These effects are mediated by the PTH receptor.
• Calcium lost through breast milk is 210 to 400 mg/d and as high as 1000 mg/d when breast-feeding twins. Mammary-derived PTHrP acts synergistically with low estradiol levels to drive bone resorption and renal calcium retention to adapt to the significant calcium demand (21,22).
• Following delivery, intestinal calcium uptake is reduced to nonpregnant levels, PTH is suppressed, and serum phosphorus increases. Calcitonin increases the first 6 weeks of the postpartum period, possibly playing a role in protecting the maternal skeleton from excessive resorption (21,22).
HYPERPARATHYROIDISM
Background
Definition
• Primary hyperparathyroidism: overproduction of PTH from adenomatous or hyperplastic parathyroid glands resulting in hypercalcemia
• Secondary hyperparathyroidism: a physiologic parathyroid response to low serum calcium concentration to maintain homeostasis
Differential Diagnosis and Etiology
• A single, benign parathyroid adenoma is the most common cause of primary hyperparathyroidism (80% to 90%). Parathyroid hyperplasia (10% to 20%) and carcinoma (less than 1%) are other primary causes.
• Consider multiple endocrine neoplasia (MEN1 or MEN2A) in patients with multigland hyperplasia on surgical pathology.
• Hypercalcemia can be due to other causes such as chronic use of lithium, hematologic malignancies, vitamin D or A toxicity, excess 1,25 vitamin D production from granulomatous disease, hyperthyroidism, Addison disease, use of thiazide diuretics, and giant mammary hyperplasia and solid tumors that produce PTHrP.
• Familial hypocalciuric hypercalcemia is an autosomal dominant condition caused by an inactivating mutation in the calcium-sensing receptor, resulting in hypercalcemia with normal PTH levels and low urinary calcium excretion. Patients are usually asymptomatic, and the key to diagnosis is the presence of family members with similar biochemistries. Homozygous mutations can cause severe neonatal hypercalcemia (22).
• Ingestion of high amounts of calcium, especially in patients with transient renal insufficiency, can impact serum calcium (milk-alkali syndrome).
Diagnosis and Evaluation
History and Physical
• The vast majority of patients are asymptomatic with mildly elevated calcium levels.
• Signs and symptoms related to hypercalcemia are more evident with levels greater than 12 mg/dL. Presentation may include fatigue, shortened QT intervals, arrhythmias, abdominal pain, constipation, peptic ulcer disease, weakness, mental confusion, skeletal pain, nephrolithiasis, or renal impairment with high serum calcium and phosphate levels.
Diagnostic Testing
• Elevated ionized calcium and inappropriately normal or elevated intact PTH in the presence of increased urinary calcium levels are observed. The use of ionized calcium compensates for the dilutional effects of pregnancy on total calcium, and the measure of intact PTH is highly specific for the bioactive hormone.
• High-resolution ultrasound imaging of the parathyroid glands can assist in the diagnosis with 64% to 85% sensitivity reported. Imaging with isotopic scanning can be useful but should be avoided in pregnancy.
Pregnancy Complications
• Maternal complications occur in up to 67% of pregnancies complicated by primary hyperparathyroidism. Perinatal complications may result in a spontaneous abortion, fetal growth restriction, intrauterine or neonatal demise, low birth weight, prematurity, hypertensive crisis, preeclampsia, nephrolithiasis, hyperemesis, pancreatitis, and rarely life-threatening consequences of severe hypercalcemia (21,23).
• Neonatal complications have been shown to be as high as 80%. Chronically high levels of maternal calcium can suppress fetal PTH and cause hypocalcemia with or without tetany or seizures in over 50% of neonates. Permanent hypoparathyroidism from impaired fetal parathyroid development is rare.
Treatment
• Surgical intervention is the definitive management for primary hyperparathyroidism. However, inherent surgical risks must be taken into consideration with maternal and fetal risks and gestational age at diagnosis. With the known morbidity and mortality associated with hypercalcemia and high fetal loss rate in pregnancies with high serum calcium, surgical intervention is recommended for women with serum calcium levels greater than 11.0 mg/dL or worrisome symptoms. Surgery is optimal mid-second trimester of pregnancy.
• Conservative medical treatment of hyperparathyroidism requires adequate fluid hydration and correction of electrolyte abnormalities (21,24).
• Correct dehydration due to calciuresis with oral and intravenous (IV) fluid.
• Once hydration is assured, furosemide may increase urinary calcium loss.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


