Study
No. EACA tested
% cases positive
Any HPV (%)
HPV 16 (%)
HPV 18 (%)
HPV 45 (%)
HPV other (%)
Li (2011)
3525
82
36.3
36.8
5.2
21.7
De Sanjose (2010)
760
62
50
32
12
6
Tornesello (2011)
39
72
57
18
7
18
An (2005)
135
90
42
36
1
21
Clifford (2008)
2521
80.3
35.3
37.9
5.6
21.2
Precursor Lesions (Adenocarcinoma In Situ, Usual Type or High-Grade Cervical Glandular Intraepithelial Lesion (HG-CGIN))
The prototypical cervical glandular neoplastic lesion is adenocarcinoma in situ (AIS) of the usual type. In the British literature, AIS has also been named “high-grade cervical glandular intraepithelial neoplasia” (HG-CGIN). These terms are now considered synonyms in the newest WHO classification system, which also includes stratified mucin-producing intraepithelial lesion (SMILE) as a variant of AIS/HG-CGIN [19]. AIS was originally described in 1953 by Friedell and McKay, and its histological features were recognized at that time [20]. The cytological morphology was described much later in the 1970s and 1980s [21–23]. There is significant evidence that AIS is a true precursor lesion to invasive carcinoma. AIS has a mean age which is reported to be from 12 to 18 years earlier than that of invasive endocervical adenocarcinoma [22, 24–26]. AIS is also more prevalent than invasive carcinoma indicating a larger pool of AIS from which only a portion goes on to develop invasive disease. AIS shows diffuse p16 immunostaining completely analogous to invasive carcinoma indicative of a neoplastic transformation of the epithelium and is positive for hrHPV of similar types to those seen in invasive endocervical cancer [27, 28]. In addition, areas of AIS are very commonly found adjacent to invasive endocervical adenocarcinomas. The usual type of AIS, in parallel with its invasive counterpart, is by far the most common in situ adenocarcinoma of the endocervix. In comparison to the other recognized in situ adenocarcinoma variants, namely, endometrioid and mucinous, the usual type constitutes more than 90% of all cases. AIS is generally not visible on colposcopic examination and may not be associated with symptoms or have only minor symptoms such as abnormal vaginal discharge [29, 30]. It is therefore most commonly identified via Pap or hrHPV testing [31–33]. Pap testing (as described below) can show diagnostic features of AIS but just as commonly shows atypical glandular cells which may be insufficient for a definitive interpretation of AIS. This equivocal finding is recognized to be very important for patient management and has been well incorporated into current management guidelines [34]. All cases of atypical glandular cells on a Pap test should prompt a colposcopic examination and histological sampling of the endocervical canal. Recent improvements in Pap test sampling device technology, which have allowed greater sampling of the endocervical canal and better recognition of the diagnostic cytological features, are thought to be the reason for the increased prevalence of AIS discovered today in screening programs. hrHPV testing is also sensitive for AIS; however, the specificity is less than with Pap testing because of the high prevalence of benign hrHPV infections in the background population. AIS most often grows in a contiguous fashion, and therefore during excisional procedures, clear margins do generally indicate complete removal [35]. However, rare examples of discontinuous AIS have been reported, and clinical follow-up following excision is necessary. Because virtually all AIS of the usual type is associated with hrHPV types, testing for hrHPV can be helpful in assessing residual disease following excision. Fortunately, complete excision of AIS is curative.
Histopathology [36, 37]
The normal endocervical epithelium adjacent to the cervical transformation zone consists of a simple columnar epithelium with basal nuclei and a mucous cap of frothy cytoplasm (Fig. 8.1). AIS manifests as a replacement of the normal endocervical epithelium by neoplastic glandular cells without evidence of invasion through the basement membrane. When AIS replaces the normal endocervical epithelium, the simple epithelium is transformed to a pseudostratified epithelium in which the mucous cap is diminished with a much increased nucleus to cytoplasm ratio. This appearance is often referred to as “mucin-poor.” At low magnification examination, the first histopathological clue may be the density of the nuclei in the epithelium which lends a hyperchromatic (dark) appearance to the surface epithelium (Fig. 8.2). Usually areas where the hyperchromatic epithelium directly abuts normal endocervical epithelium are noted which accentuates the stark contrast between the normal and neoplastic epithelia (Fig. 8.3). At high magnification, the cells of AIS show enlarged nuclei, on average about two times the size of normal endocervical cell nuclei. The nuclei are elongate and show significant overlapping in the pseudostratified areas. Nucleoli are present but may not always be prominent. Nuclear chromatin is typically coarsely granular and evenly distributed, and nuclear envelops are irregular. Mitotic figures are common, and apoptotic debris (nuclear breakdown fragments indicative of cell turnover) is also common (Fig. 8.4). Architecturally, AIS does not generally show areas of solid or cribriform growth. If such areas are identified, a careful examination of the specimen for early invasion is indicated.





Fig. 8.1
Normal endocervical epithelium in the area of the transformation zone is simple, columnar, and non-stratified. Uniform nuclei are present in the basal portion of the cell, and the luminal columnar cytoplasm contains frothy mucus (hematoxylin and eosin stain, high magnification)

Fig. 8.2
Endocervical adenocarcinoma in situ is most often initially noted during low magnification scanning due to the hyperchromasia of the stratified nuclei in comparison to the pallor of the normal mucus-rich endocervical epithelium (hematoxylin and eosin stain, low magnification)

Fig. 8.3
Endocervical adenocarcinoma in situ commonly shows a sharp margin with adjacent normal endocervical epithelium. Note the very clear demarcation between lesional and normal columnar epithelium (arrow). AIS shows prominent pseudostratification with loss of the mucus cap compared to the normal simple architecture with prominent frothy mucus. Note the prominent mitotic activity (arrow head) (hematoxylin and eosin stain, high magnification)

Fig. 8.4
The nuclei of endocervical adenocarcinoma in situ are typically about two times the size of normal endocervical cell nuclei. Apoptotic debris is commonly present in neoplastic endocervical epithelium and is indicative of increased cell turnover (arrow) (hematoxylin and eosin stain, high magnification)
Cytopathology [37–39]
The cytopathological appearance of AIS recapitulates the histopathology closely. Cytological specimens from patients with AIS may show abundant individual endocervical cells and hyperchromatic crowded groups (HCGs) of endocervical cells. Any specimen containing abundant endocervical material should be examined very carefully for the presence of abnormality within these cells. However, overall cellularity is dependent on the sampling of the lesion and may show few abnormal cells if the lesion is small, high in the canal, or not directly brushed. On initial low magnification examination, the hyperchromatic crowded groups typically show nuclear and cytoplasmic protrusion at the group margins. This phenomenon is referred to as “feathering” and can be seen in any endocervical proliferation but is particularly accentuated in AIS. In conventionally prepared specimens, feathering is most prominent due to flattening of the groups during the smearing process (Fig. 8.5). In liquid-based specimens, where groups are more three-dimensional, feathering can be more subtle, but is still present in most cases. The sentinel finding of AIS is the presence of strips of pseudostratified columnar epithelium with depletion of the mucous cap on the luminal portion of the cell (Fig. 8.6). Polarity of the cells with identification of a basal and luminal aspect of the strip is important in order to discriminate AIS from high-grade squamous intraepithelial lesion (HSIL) (see Chap. 7) growing into a gland. In the latter, an appearance of a pseudostratified epithelium without a luminal-basal orientation is a key to that interpretation (Fig. 8.7). Other dense groups of glandular cells with high nucleus to cytoplasm ratio and hyperchromatic nuclei (HCGs) are also usually present. Often partial or complete gland formations (epithelial “rosettes”) are noted in association with the HCGs (Fig. 8.8). Individual abnormal cells showing columnar configuration and atypical nuclei can be found distributed across the slide (Fig. 8.9). The individual cells show nuclei that are about two times the size of a normal endocervical cell. Nuclei are hyperchromatic with granular chromatin which is evenly distributed. Uneven chromatin distribution (so-called chromatin clearing or chromatin heterogeneity) should prompt a consideration of invasive endocervical adenocarcinoma. Nucleoli are present, but not generally prominent. Mitotic figures and apoptotic debris are frequently identified. The background of the slide does not show a tumor diathesis, which is caused by tissue destruction and tumor necrosis, both of which are not present in AIS and if present should also suggest the possibility of an invasive carcinoma. The background may show an increased number of acute inflammatory cells.






Fig. 8.5
Even at low magnification, groups of cells showing marginal protrusion of nuclei and cytoplasm (“feathering”) is characteristic of endocervical adenocarcinoma in situ and may be the first indication of a neoplastic process (arrows). Feathering tends to be more prominent in conventionally prepared specimens due to flattening of the groups during the smearing and fixation process

Fig. 8.6
In cytological specimens, pseudostratified strips of cells are a key feature of endocervical adenocarcinoma in situ. Note the coarse granularity with even distribution of the nuclear chromatin in this example (arrow) (Papanicolaou stain, medium magnification)

Fig. 8.7
A mimic of endocervical adenocarcinoma in situ on cytology can be seen in crowded groups of high-grade squamous intraepithelial lesion (HSIL) involving an endocervical gland (Papanicolaou stain, medium magnification)

Fig. 8.8
In addition to pseudostratified strips of cells and feathering, endocervical adenocarcinoma often shows full or partial rosette arrangements of cells, indicative of gland-like formations (arrow) (Papanicolaou stain, medium magnification)

Fig. 8.9
In addition to crowded groups of cells, cases with endocervical adenocarcinoma in situ typically show atypical individual cells scattered in the background. The cells are tall and slender, with enlarged nuclei and coarse but evenly distributed nuclear chromatin (arrow) (Papanicolaou stain, high magnification)
Invasive Endocervical Adenocarcinoma, Usual Type
The usual type of invasive endocervical adenocarcinoma, just as in its correlate precursor lesion, is the most common type of cervical adenocarcinoma. It generally is recognized as comprising 80–90% of all adenocarcinomas, although several recent reports have shown that in Japanese populations, mucinous adenocarcinomas of gastric type may comprise as much as 30% of the total [40]. As in AIS, early superficially invasive adenocarcinomas may present with no symptoms, or occasionally with only minor symptoms, such as abnormal discharge or bleeding. In this early stage, Pap or HPV testing is most likely to show the only abnormal initial findings. Colposcopy may show areas of abnormality when the lesion occupies the lower portion of the endocervical canal. In larger tumors, symptoms of abnormal bleeding are almost always present and lesions are grossly visible on colposcopic examination. In larger tumors, the Pap test nearly always shows abnormality which may be present as either a diagnostic appearance of carcinoma or as atypical glandular cells.
The prognosis of invasive cervical adenocarcinoma is dependent on stage at presentation, with low stage disease generally having a good (curative) outcome (Tables 8.2 and 8.3). Most studies have shown that stage for stage cervical adenocarcinoma has a worse prognosis than squamous cell carcinoma [41–45]. Differences in dissemination and recurrence have been found for endocervical adenocarcinoma versus squamous cell carcinoma. Ovarian metastases are more common in endocervical adenocarcinoma than in squamous cell carcinoma [46]. Higher rates of distant metastasis have also been noted for endocervical adenocarcinoma [42, 47].
Table 8.2
Staging of cervical adenocarcinoma (TNM and FIGO)
TNM categories | FIGO stages | Definitions |
|---|---|---|
Primary tumor (T) | ||
Tx | Primary tumor cannot be assessed | |
T0 | No evidence of primary tumor | |
Tis | Carcinoma in situ (preinvasive carcinoma) | |
T1 | I | Cervical carcinoma confined to the uterus (ignore corpus extension) |
T1a | IA | Invasive carcinoma diagnosed on microscopy only |
T1a1 | IA1 | Stromal invasion ≤3.0 mm in depth, ≤ 7.0 mm in width |
T1a2 | IA2 | Stromal invasion 3.0–5.0 mm in depth, ≤ 7.0 mm in width |
T1b | IB | Clinically visible tumor confined to cervix or microscopic size > T1a/IA2 |
T1b1 | IB1 | Clinically visible tumor ≤4.0 cm |
T1b2 | IB2 | Clinically visible tumor >4.0 cm |
T2 | II | Cervical carcinoma invades beyond the uterus but not to the pelvic wall or lower one third of vagina |
T2a | IIA | No parametrial invasion or involvement of the lower one third of the vagina |
T2a1 | IIA1 | Clinically visible lesion ≤4.0 cm involving < the upper two third of the vagina |
T2a2 | IIA2 | Clinically visible lesion >4.0 cm involving < the upper two third of the vagina |
T2b | IIB | Tumor with parametrial invasion |
T3a | IIIA | Tumor involves the lower one third of the vagina, no extension to the pelvic wall |
T3b | IIIB | Tumor extends to the pelvic wall and/or causes hydronephrosis or nonfunctioning kidney |
T4 | IVA | Tumor invades the mucosa of the bladder or rectum and/or extends beyond the true pelvis |
Regional lymph nodes (N) | ||
NX | Regional lymph nodes cannot be assessed | |
N0 | No regional lymph node metastasis | |
N1 | Regional lymph node metastasis | |
Distant metastasis (M) | ||
M0 | No distant metastasis | |
M1 | IVB | Distant metastasis |
Table 8.3
Anatomical stage/prognostic groups of cervical adenocarcinoma
Anatomical stage/prognostic groups | |||
|---|---|---|---|
Stage 0 | Tis | N0 | M0 |
Stage I | T1 | N0 | M0 |
Stage II | T2 | N0 | M0 |
Stage IIIA | T3 | N0 | M0 |
Stage IIIB | T1–3 | N1 | M0 |
Stage IVA | T4 | Any N | M0 |
Stage IVB | Any T | Any N | M1 |
Histopathology [36, 37]
The usual type of endocervical adenocarcinoma is typically of moderate differentiation but can also present as well- or poorly differentiated lesions. In the best differentiated lesions, particularly in cases showing only superficial invasion, it may be difficult to distinguish between AIS and invasive cancer. Involvement of endocervical glandular structures that are below the level in the cervical wall of normal endocervical glands and changes in the stromal tissue surrounding the abnormal glands, such as inflammation, myxoid change, or “swirling” of stromal fibroblasts around the nests of neoplastic cells, are clues to superficial invasion (Fig. 8.10). In addition, changes in the neoplastic cells compared to those directly adjacent to the areas of suspected invasion can also present a clue to invasion. Increased amounts of cytoplasm and the presence of more prominent macronucleoli can be seen in association with increase in metabolic activity necessary to penetrate the basement membrane and spread into the stromal tissues (Fig. 8.11). In these superficially invasive lesions, the neoplastic cells replace the normal endocervical cell lining of the glands. In distinction to the pseudostratified architecture of AIS, early invasive carcinoma may also show more complex architecture including solid patterns and areas of cribriform growth. Identification of either of these two architectural features should prompt consideration of an invasive tumor.

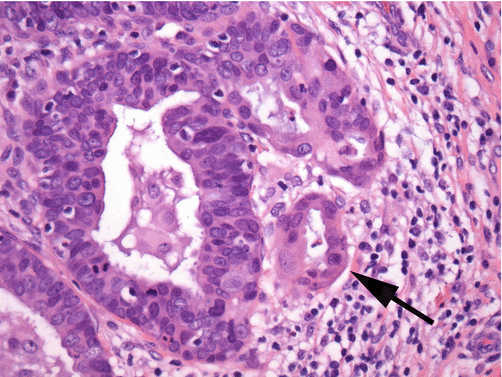

Fig. 8.10
Superficially invasive endocervical adenocarcinoma shows markedly irregular glandular spaces which impinge on the adjacent normal endocervical glands. Stromal reaction and inflammation often surrounds the early invasive nests (arrow) which is a key diagnostic feature differentiating an invasive from an in situ lesion (hematoxylin and eosin stain, low magnification)

Fig. 8.11
Two early clues to the presence of invasion are stromal reaction and changes in cytoplasm. Glands surrounded by an edematous or inflamed swirling stroma or an increase in cytoplasmic volume with eosinophilia (arrow) are highly associated with invasive disease (hematoxylin and eosin stain, high magnification)
In clearly identifiable invasive endocervical adenocarcinoma, the abnormal glands are small to medium sized and penetrate the cervical wall to various levels, inciting an obvious stromal response (Fig. 8.12). Gland lumina, when discernible, may show necrotic debris. Occasional cystic glands may be present with mucin within the gland lumen or occasionally free in the stromal tissue. The abnormal cells retain a columnar appearance, with either pseudostratified, solid/cribriform, or occasionally papillary tufted growth pattern. The individual cells have high nucleus to cytoplasm ratios with granular “mucin-poor” cytoplasm. Nuclei are enlarged at greater than two times the size of normal endocervical cell nuclei and can range in shape from tall fusiform to oval. Nuclear contours are irregular. The nuclei are hyperchromatic having dense granular cytoplasm and areas of chromatin heterogeneity (so-called chromatin “clearing”) (Fig. 8.13). Numerous mitotic figures are noted, often near the apex of the cells giving the appearance of “floating mitoses” (Fig. 8.14). Apoptotic debris is also commonly noted.


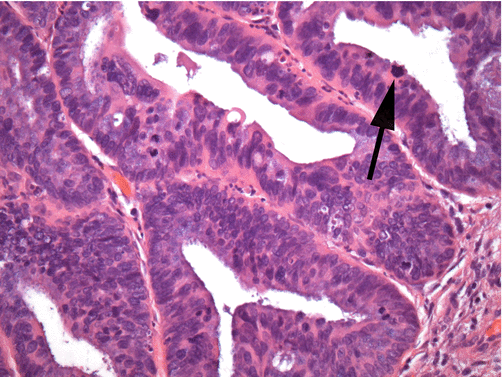

Fig. 8.12
Invasive endocervical adenocarcinoma of the usual type commonly shows cribriform architecture with some foci showing a solid growth pattern (hematoxylin and eosin stain, medium magnification)

Fig. 8.13
The nuclei of endocervical adenocarcinoma of the usual type are typically pleomorphic with irregularities of size and shape. Note the coarse granularity of the chromatin which in contrast to in situ lesions shows areas of heterogeneity (arrow) (hematoxylin and eosin stain, high magnification)

Fig. 8.14
“Floating” mitoses which are present near the luminal surface of the cells are common in endocervical adenocarcinoma of the usual type (arrow) (hematoxylin and eosin stain, high magnification)
Cytopathology [37–39]
The cytopathological appearance of invasive adenocarcinoma, similar to the histopathology, is dependent on the degree of differentiation of the tumor. Well-differentiated lesions may be indistinguishable from AIS. As the tumors become less differentiated, the amount of sampled neoplastic cellular material increases and the appearance of the cells becomes more atypical. Adenocarcinoma presents as isolated atypical cells, as pseudostratified strips of cells, as dense hyperchromatic crowded groups, and as two-dimensional sheets of cells (Fig. 8.15). The difference between the latter two presentations depends on how the sampling of the cells took place. Two-dimensional groupings mean the cells were directly sampled from the tumor surface and recapitulate their in situ appearance; while three-dimensional groups imply that the cells were spontaneously exfoliated prior to being sampled (Fig. 8.16). Exfoliation allows for cell groupings (and individual cells) to round up as they “float” in the cervical mucus. The individual cells of adenocarcinoma show high nucleus to cytoplasmic ratios with granular “mucin-poor” cytoplasm. The nuclei are oval to fusiform, hyperchromatic with dense coarsely granular chromatin showing areas of heterogeneity (“clearing”), and prominent macronucleoli (Fig. 8.17). Mitotic figures and apoptotic debris are commonly present. The slide background commonly shows the presence of granular cellular breakdown material intermixed with inflammatory cells (so-called tumor diathesis) which is indicative of tissue necrosis and inflammatory response. In conventionally prepared cytology specimens, the diathesis material is spread evenly in the background of the slide, while in liquid-based cytology specimens, the diathesis material may aggregate and cling to the surface of cells (Fig. 8.18).
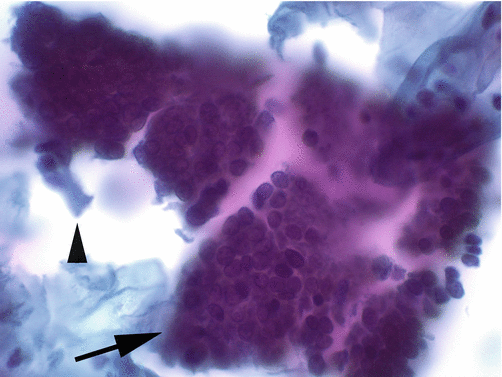




Fig. 8.15
Endocervical adenocarcinoma of the usual type presents as groups and as isolated cells. Groups retain features of columnar epithelia with “honeycomb” architecture (arrow), and isolated cells recapitulate the columnar configuration of normal endocervical cells (arrowhead) (Papanicolaou stain, high magnification)

Fig. 8.16
The cytological presentation of endocervical adenocarcinoma of the usual type depends on the method of sampling. When directly sampled, a two-dimensional sheet of cells is present (a) (Papanicolaou stain, medium magnification). And when tumor cells exfoliate prior to sampling, three-dimensional clusters are the norm (b) (Papanicolaou stain, high magnification)

Fig. 8.17
The nuclei of endocervical adenocarcinoma of the usual type show heterogeneous coarse chromatin granularity and prominent nucleoli (Papanicolaou stain, high magnification)

Fig. 8.18
Tumor diathesis consisting of granular amorphous debris is commonly found in the background and clinging to the surface of intact cells in invasive carcinoma (arrow) (Papanicolaou stain, medium magnification)
Immunohistochemistry of Endocervical Neoplasia
p16 immunohistochemistry is a useful marker for the presence of a true endocervical neoplastic lesion of the usual type. Virtually all usual type AIS and invasive endocervical adenocarcinomas are hrHPV positive and will show aberrant accumulation of p16. There are caveats for interpretation of this stain however. A positive stain should only be considered one in which the entire epithelium is diffusely positive, most often with both nuclear and cytoplasmic staining (Fig. 8.19a). This is important because several of the benign mimics noted below, such as tubal metaplasia and endometriosis, can show incomplete spotty, but sometimes strong staining for p16 (Fig. 8.19b). A marker of increased cellular proliferation (Ki67 or Mib1) can be supportive of a neoplastic process in conjunction with p16; however, studies have shown that Ki67 adds little predictive value to the p16 assay (Fig. 8.19c). Another marker IMP3 has been shown to be more specific for AIS with diffuse staining of most usual type glandular neoplasias with little staining reported in benign processes [48].


Fig. 8.19
Immunohistochemical stains can be helpful in distinguishing neoplastic endocervical lesions from benign mimics. p16 staining is strong and diffuse in neoplasia (a), while benign tubal metaplasia shows only focal cells which are immunoreactive (b). In (a), note the p16-positive lesional tissue in comparison to the negative residual benign endocervical glands. Increased numbers of cells show reactivity with Ki67 in neoplastic lesions (c)
Immunohistochemistry can be useful in distinguishing endocervical from endometrial neoplasms, particularly when the lesions are large and extend to both cervix and corpus or are present in metastatic sites. Endocervical carcinoma is diffusely p16 positive, whereas endometrial cancer is typically only focally positive. Additionally, endocervical neoplasia is positive for CEA and negative for estrogen receptor and vimentin. Endometrial neoplasia is negative for CEA and positive for estrogen receptor and vimentin [49]. Both origins are most often positive for PAX8.
Histological Mimics of In Situ and Invasive Adenocarcinoma
Tubal and Tuboendometrioid Metaplasia
In tubal metaplasia (TM), endocervical glandular epithelium is replaced by tubal-type epithelium composed of ciliated cells, nonciliated secretory cells, and intercalated (peg) cells (Fig. 8.20). Tubal metaplasia is a common finding in the endocervical canal, being present in 21% of cone biopsies and 62% of hysterectomy specimens in a prevalence study [50] and becoming more frequent as women age [51]. Less commonly the presence of a mixture of tubal- and endometrial-type epithelia known as tuboendometrioid metaplasia (TEM) is identified. This is similar to TM but with few to rare ciliated cells (Fig. 8.21). TEM has been found in 26% of hysterectomy specimens when a prior cone biopsy had been performed, suggesting that it may be a reparative response in at least some cases [52]. Usually the glands involved by TM and TEM otherwise resemble normal endocervical glands, but one or more unusual features can occasionally be present, such as variability in size and shape, cystic dilatation, pseudostratified architecture, focal crowding, high nuclear to cytoplasmic ratio, mitotic figures, a deep location, and periglandular stromal hypercellularity or edema (Fig. 8.22) [53]. At low magnification, the dark-staining epithelia may initially raise the possibility of a well-differentiated invasive adenocarcinoma or AIS. The admixture of cell types, including the prominence of ciliated cells, as well as the lack of nuclear atypia, only rare mitotic figures without apoptotic debris, and lack of a desmoplastic stromal reaction, should allow a correct interpretation as a benign metaplastic process. Immunohistochemical staining for p16, Bcl2, and MIB1/Ki67 can help differentiate neoplastic endocervical glands from benign TM/TEM.




Fig. 8.20
Tubal metaplasia consists of a mucin-poor, pseudostratified epithelium which replaces normal endocervical epithelium in the upper canal as women age. Its hyperchromatic appearance gives an appearance of neoplasia at low magnification. Note the heterogeneity of epithelial cell types vacuolated (arrow) and ciliated (arrowhead), which are the key to a correct benign interpretation (hematoxylin and eosin stain, high magnification)

Fig. 8.21
Tuboendometrioid metaplasia is diagnosed only on histology. The glands have a mucin-depleted appearance with oval, elongate nuclei; the glands usually are surrounded by stroma with slightly increased cellularity (hematoxylin and eosin stain, low magnification)

Fig. 8.22
Tubal metaplasia can present in a crowded/solid pattern with rosette-like structures which can mimic endocervical neoplasia. The presence of luminal cilia is a key benign feature
Oxyphilic Metaplasia
Oxyphilic metaplasia is an incidental finding in cervical specimens having no clinical significance. It manifests as focal replacement of endocervical epithelium with cuboidal cells having dense, eosinophilic, and focally vacuolated cytoplasm. The nuclei are usually large, hyperchromatic, and somewhat degenerate which may give rise to erroneous considerations of in situ adenocarcinoma (Fig. 8.23). Although a certain degree of epithelial atypia has been described, unlike adenocarcinoma, oxyphilic metaplasia lacks stratified cells, marked atypia, and mitotic activity which should allow for a correct interpretation [54].


Fig. 8.23
Oxyphilic metaplasia shows cells within a metaplastic endocervical epithelium with voluminous amounts of eosinophilic granular cytoplasm. Nuclear atypia, relating to degenerative change, can be associated which can give a false impression of a neoplastic process (hematoxylin and eosin stain, high magnification)
Endometriosis
Similar to tubal and tuboendometrioid metaplasia discussed above, endometriosis consists of ectopic endometrial-type glandular epithelium with the addition of endometrial stroma (Fig. 8.24). Endometriosis can occur either superficially involving the endocervical mucosa and glands or more deeply in the cervical wall. The superficial form may result following trauma (e.g., cone biopsy) or as a result of implantation due to menstruation [55]. Deep endometriosis often occurs as a part of more widespread pelvic endometriosis. Most often endometriosis resembles proliferative phase endometrium. Correct recognition is not difficult if the stroma and glands are in the usual proportion, but if endometrial glands predominate, the diagnosis of adenocarcinoma may be entertained due to the less abundant cytoplasm, nuclear stratification, and scattered mitotic figures that are a normal part of the proliferative cycle. A diagnostic clue in favor of nonneoplastic endometriosis is the presence of small arterioles hugging the glands within the scant stroma. Immunohistochemical stains may also help; endometrial stromal cells are positive for CD10 and negative for CD34, while the reverse is the case for endocervical stromal cells. In addition, endometriotic glands are positive for Bcl2, while endocervical glands are negative [56]. Occasionally only endometrial stroma will be identified and should not be misinterpreted as a far less common sarcoma [57].


Fig. 8.24
Endometriosis involving the cervix consists of endometrial-type glands and stroma, often with hemorrhage. The pseudostratified architecture of endometrial epithelium, along with mitoses, and occasional apoptotic debris makes it a good mimic of endocervical neoplasia (hematoxylin and eosin stain, medium magnification)
Endocervicosis
Benign-appearing endocervical glands may rarely be located deep in the cervical wall (Fig. 8.25). This condition most commonly gives rise to a diagnostic consideration of well-differentiated mucinous adenocarcinoma because of the normal apical mucus. If the deep endocervical glands show any features of tubal or tuboendometrioid metaplasia, a consideration of the usual type of endocervical adenocarcinoma may also be considered, primarily due to the inherent pseudostratification with hyperchromasia [58]. The absence of malignant nuclear features and an in situ component at the mucosal surface provides solid evidence against malignancy.




