Class I
Negative
Absence of atypical or abnormal cells
Class II
Negative
Atypical cells present but without abnormal features
Class III
Suspicious
Cells with abnormal features suggestive but not conclusive for malignancy
Class IV
Positive
Cells and cell clusters fairly conclusive for malignancy
Class V
Positive
Cells and cell clusters conclusive for malignancy
The entity of carcinoma in situ, the immediate precursor lesion of invasive squamous cell carcinoma of the cervix, in which the constituent cells morphologically looked like the cells found in invasive squamous cell carcinoma, had been recognized from the late nineteenth century [41–44]. However by the early 1950s, surface lesions of the cervix with abnormal but less marked histological features had been identified, for which a number of terms were suggested including anaplasia, basal cell hyperplasia, atypical metaplasia, and atypical hyperplasia. In 1953 Regan proposed the term dysplasia, from the Greek dys, bad, and plasia, molding, which he divided into three grades, mild, moderate, and severe. This proposal was endorsed by the First International Congress of Exfoliative Cytology and the World Health Organization: in the latter the abnormal cells were described in terms of their histological correlation [45, 46]. Dysplasia appeared to have a lower risk of progression to cancer than carcinoma in situ, and consequently, at that time, women found to have carcinoma in situ were recommended to have a hysterectomy, while those with dysplasia were not immediately treated [47, 48].
During the establishment of the cervical screening program in the UK, it became apparent that a variety of terminology was being used to describe the morphological appearances of neoplastic cells derived from in situ and invasive cervical squamous lesions. In particular the practice in many laboratories of calling cells thought to be derived from carcinoma in situ “malignant cells” and using “dyskaryosis” to imply that nothing more than dysplasia was present began to be questioned in the light of the conclusive evidence from Richart that dysplasia and carcinoma in situ of the cervix were a “lesional continuum” [49]. A working party of the British Society for Clinical Cytology (BSCC) recommended that the terminology in the WHO publication Cytology of the Female Genital Tract be adopted for normal cellular components of a cervical smear (e.g., superficial, intermediate, and parabasal squamous cells; endocervical cells; endometrial cells) and the term “dyskaryosis” adopted for neoplastic squamous and glandular cells, irrespective of whether the cytologist thought that they were derived from an in situ or invasive lesion [50] (Table 3.2).
Table 3.2
Definition of dyskaryosis
Disproportionate nuclear enlargement |
Irregularity in nuclear form and outline |
Hyperchromasia |
Multinucleation |
Irregular chromatin distribution, which may be stippled, clumped, or stranded with condensation beneath the nuclear membrane |
Abnormalities of the number, size, and form of nucleoli |
Eight years later, a second BSCC working party endorsed the recommendation of dyskaryosis as the preferred terminology and recommended a three-grade system of mild, moderate, and severe dyskaryosis, based on the nuclear-cytoplasmic area of the dyskaryotic cells, which correlated with cells from the surface of CIN 1, CIN 2, and CIN 3, respectively. They also provided guidance on cytological features which were suggestive of the presence of invasive squamous carcinoma. This recommendation was universally adopted in the UK cervical cancer screening programs [51] (Table 3.3).
Table 3.3
BSCC terminology in gynecological cytopathology (1986)
Grade | Morphological features | Histological correlate |
|---|---|---|
Mild dyskaryosis | The abnormal nucleus occupies less than half the area of the cell, which has plentiful thin translucent cytoplasm with angular borders resembling a superficial or intermediate squamous cell | CIN 1 |
Moderate dyskaryosis | The abnormal nucleus occupies one half to two-thirds of the area of the cell. There is more disproportionate nuclear enlargement than in mild dyskaryosis, and nuclear morphology tends to be more abnormal than in mild dyskaryosis. The cytoplasm resembles that of intermediate, parabasal, or superficial cells. | CIN 2 |
Severe dyskaryosis | The abnormal nucleus practically fills the cell or at least two-thirds of its area and is surrounded by a narrow rim of thick dense cytoplasm. Affected cells may be round, oval, elongate, or polygonal | CIN 3 |
The 1986 working party also recognized that “There are smears in which the evidence is such that it is impossible to decide if the cells are the product of inflammation or if they have neoplastic potential” and suggested that such samples be described as showing borderline abnormalities. In 1994, a joint working party of the National Health Service Cervical Screening Programme (NHSCSP), the BSCC, and Royal College of Pathologists provided guidance on the diagnosis and management of borderline nuclear changes in squamous and glandular cells and their distinction from reactive or inflammatory change and neoplastic change [52].
In 2002, conscious of the widespread adoption of the two-tiered Bethesda system for reporting cervical cytology, originally developed in 1988 and subsequently modified in 2001, which reflected clinical practice and management in terms of low- and high-grade abnormality, the BSCC held a conference at which it was agreed that a two-tier system should also be introduced in the NHSCSP [53–58]. The revised BSCC terminology for cervical cytology was published in 2008 [59] and implemented in the NHSCSP in 2013. This terminology aligns closely with the Bethesda system, reflects contemporary understanding of the biology of human papillomavirus (HPV) infection, and permits international comparison of data (Table 3.4). The principal change introduced by this terminology is that while dyskaryosis is retained as the descriptor of neoplastic cell nuclear morphology, it is graded by evaluation of nuclear: cytoplasmic diameter rather than area, as previous studies had shown that the former was a more reliable discriminator of mild from moderate or severe dyskaryosis, i.e. low-grade from high-grade dyskaryosis, in both conventional and liquid-based cervical cytology preparations [60].
BSCC 1986 and NHSCSP | BSCC proposed new terminology | The Bethesda system 2001 | ECTP terminology | AMBS 2004 |
|---|---|---|---|---|
Negative | Negative | Negative for intraepithelial lesion or malignancy | Within normal limits | Negative |
Inadequate | Inadequate | Unsatisfactory for evaluation | Unsatisfactory due to | Unsatisfactory |
Borderline nuclear change | Borderline change, squamous, but not otherwise specified | Atypical squamous cells of undetermined significance (ASC-US) | Koilocytes (without changes suggestive of intraepithelial neoplasia) Squamous cell changes (not definitely neoplastic but merit early repeat) | Possible low-grade squamous intraepithelial lesion |
Borderline change, high-grade dyskaryosis not excluded | ASC-H (cannot exclude HSIL) | Possible high-grade squamous intraepithelial lesion | ||
Borderline change in endocervical cells | Atypical endocervical, endometrial, or glandular (NOS or specify in comments) Atypical endocervical or glandular cells, favor neoplastic | Atypical glandular cells (qualify) | Atypical endocervical cells of undetermined significance Atypical glandular cells of undetermined significance | |
Mild dyskaryosis | Low-grade dyskaryosis (includes all cases of koilocytosis provided that no high-grade dyskaryosis is present) | Low-grade squamous intra-epithelial lesion (LSIL) | Mild dysplasia (CIN1) | Low-grade squamous intraepithelial lesion |
Moderate dyskaryosis | High-grade dyskaryosis | High-grade squamous intra-epithelial lesion (HSIL) | Moderate dysplasia (CIN2) | High-grade squamous intraepithelial lesion |
Severe dyskaryosis | HSIL | 1. Severe dysplasia (CIN3) 2. Carcinoma in situ (CIN3) | ||
Severe dyskaryosis? invasive | High-grade dyskaryosis? invasive | Squamous cell carcinoma | 1. Severe dysplasia? invasive 2. Invasive squamous cell carcinoma | Squamous cell carcinoma |
? Glandular neoplasia | ? Glandular neoplasia, endocervical, non-cervical | 1. Endocervical carcinoma in situ 2. Adenocarcinoma – endocervical, endometrial, extrauterine, not otherwise specified | Adenocarcinoma AIS, endocervical, endometrial, extrauterine NOS | Endocervical adenocarcinoma in situ Adenocarcinoma |
The NHS Cervical Screening Program (1986–2004)
Despite the establishment of the cervical screening program as described above, it was clear by the mid-1980s that it had had little impact on the incidence or mortality from cervical cancer. In 1985 a leading article in The Lancet drew attention to this fact and specifically commented that the most successful cancer screening programs are organized as public health cancer control programs, specifically directed toward a reduction of mortality; call the age group at greatest and most immediate risk (30 years +) based on population registers and keep on trying to call persistent non-attenders; concentrate first upon women who have never had a smear; and put “someone in charge” (a manager) of the process who can be held to account [61]. In 1988 health circular HC (88)1 directed District Health Authorities to give priority to screening for prevention of cervical cancer and in particular implementation of a call and recall system from lists of women held on Family Practitioner Committee (primary care) computers starting not later than 31 March 1988. All women aged 20–64 were to be invited for screening at least every 5 years (some health authorities elected to invite women every 3 years) and adequate facilities made available for prompt investigation treatment and follow-up of women with abnormal smear results [62]. General practitioners were also offered a financial incentive based on the proportion of their practice female population eligible for cervical screening that were tested. Initially the NHS cervical screening program was managed by a multidisciplinary National Coordinating Network but subsequently a director, Professor Julietta Patnick, and support staff were appointed in 1994 [63, 64]. Over the succeeding two decades, in collaboration with the relevant professional bodies, the NHSCSP produced a comprehensive series of guidance documents related to all aspects of the cervical cancer screening process from invitation to attend screening to treatment of identified abnormality. In particular, the first NHSCSP commissioned guidance entitled Achievable standards, benchmarks for reporting and criteria for evaluation and, thereby henceforth known as ABC 1, gave guidance on specimen adequacy, management of smear abnormality, evaluation of the program, internal quality control (IQC), and external quality assurance (EQA) [65] (Table 3.5). In relation to IQC and EQA, ABC 1 introduced achievable standard ranges for cytology reporting by laboratories and individuals, and in subsequent years these ranges were amended based on the mandatory returns (KC61) submitted by laboratories in the preceding year (Table 3.6). In the first of two subsequent editions of ABC, published in 2000, guidance on reporting of cervical smears was reinforced and where necessary revised, new performance indicators were introduced, and pitfalls in cytological diagnosis leading to false-positive and false-negative results described [66, 67]. In the second subsequent edition of ABC, published in 2013, adoption of the revised BSCC terminology for cervical cytology was mandated, management of cytological abnormality updated following the implementation of HPV triage and test of cure, and performance indicators for evaluating cervical cytopathology expanded to encompass not only individual and laboratory cytology performance but also the performance of related colposcopy and histopathology services [68–70].
Table 3.5
ABC 1: recommendations for management
Management | Cytology result |
|---|---|
Routine recall | Negative |
Repeat smear at shorter interval than recommended routine recall | Inadequate sample and the first occurrence of mild dyskaryosis or borderline change A second repeat sample may be requested for inadequate samples or borderline change, but after three such smears colposcopy must be recommended. The repeat interval may vary between 3 and 12 months but is usually 6 months Annual repeat smears are recommended for 5 years after treatment of CIN 2 and CIN 3 At least two negative smears at least 6 months apart, after mild dyskaryosis, borderline change or treatment of CIN 1 before a woman returns to routine screening or screening is ceased at age 65 |
Referral for gynecological opinion | Moderate, severe, and ungraded dyskaryosis; invasive squamous and glandular neoplasia should all be referred on the first occurrence. Colposcopy should be recommended on the second occurrence of mild dyskaryosis |
Table 3.6
ABC 1: criteria for evaluating cervical cytology and monitoring the accuracy of screening
Measurement | Achievable range |
|---|---|
Sensitivity of primary screening with respect to the final report after rapid review of all negative and inadequate smears | >90% all abnormalities >95% high-grade abnormality |
Laboratory report profile: Inadequate Mild dyskaryosis and borderline change Moderate and severe dyskaryosis | 7.0 ± 2.0% 1.6 ± 0.4% 5.5 ± 1.5% |
Positive predictive value (PPV) of moderate or severe dyskaryosis for the histological diagnosis of CIN 2 or worse | 65–85% |
The success of the reorganized English cervical screening program as NHSCSP was evidenced by the progressive fall in incidence of cervical cancer in the succeeding two decades: this has now largely stabilized (Fig. 3.1). The increased incidence around 2009 was the result of the increased uptake of screening due to the widely publicized diagnosis and death of a television personality [71, 72].


Fig. 3.1
Age-standardized incidence rates of invasive cervical cancer in the UK (1979–2013) (Source: Cancer Research UK. http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/cervical-cancer. Accessed August 2016)
NHSCSP 2004 to the Present
Liquid-Based Cytology (LBC)
From the late 1980s, a number of manufacturers began to investigate the potential to produce monolayer or near-monolayer preparations of cervical cytology samples, with the intention that this would provide an optimized platform on which to employ computer-assisted image analysis microscopy. Production of near-monolayer preparations required samples to be collected in a liquid preservative – hence liquid-based cytology – and then most of the debris, blood, and exudate removed either by filtration or density gradient sedimentation, prior to preparation of the monolayer or near-monolayer sample. By the late 1990s, two systems were widely available: ThinPrep® (Hologic) and SurePath® (BD Diagnostics). In 2000 an initial evaluation by the National Institute for Clinical Excellence (NICE) suggested that LBC might be valuable technology to implement in the NHSCSP [73]. In 2003, following a further evaluation by NICE and an evaluation study in three English laboratories, the Department of Health (DoH) announced that LBC was to be used as the primary means of processing samples in the cervical screening program in England and Wales and full implementation of the new technology was to be achieved by 2008 [74, 75]. Implementation was conducted by a cascade process of laboratory conversion and training, and by late 2008 all cervical screening laboratories in England and Wales had converted to LBC. Scotland had already adopted LBC and Northern Ireland followed some time later [76].
Importantly, at the same time the DoH also announced changes in screening age range and frequency to be implemented by April 2004: women would in future be invited for their first screening test at age 25, not age 20, and screened thereafter every 3 years until age 49 and every 5 years from age 50 to 64 [77]. This policy change, based on an audit of the screening histories of women with invasive cervical cancer [78], was intended to unify and consolidate considerable variation in practice across England: as noted above the national recommendation was to screen every 5 years but some districts had elected to screen every 3 years. While concerns were raised about the effect of not screening women less than 25 years of age, it has been kept under review through the national audit of screening histories of women who develop cervical cancer: most cervical cancers in women under age 30 years are screen detected as superficially invasive carcinomas (FIGO stage IA) [79–83].
As predicted, progressive implementation of LBC, combined with the change in screening age range and frequency, resulted in a reduction in the number of inadequate samples reported and thereby a decrease in the total number of tests examined: over 246,000 fewer tests were reported as inadequate in 2007–2008 compared with 2003–2004, the last year before LBC implementation. This also occurred against a background of an increased number of women aged 25–64 being screened, reflecting a more efficient screening program with fewer unnecessary tests outside the recommended screening age range [84]. Furthermore, the progressive loss of tests in women aged less than 25 years reduced the number of abnormal tests reported, particularly low-grade abnormalities which are most prevalent in this age group: nearly 19,000 fewer tests were reported as low grade and over 4000 as high grade in 2007–2008 compared with 2003–2004. As a result there was reconfiguration of consultant programmed activities in some laboratories to ensure maintenance of quality standards for the minimum number of abnormal tests examined annually.
Therefore, following a change in the screening age range and frequency and full implementation of LBC, a total of 269,000 fewer cervical cytology samples were examined in England in 2007–2008 compared with 2003–2004. Implementation of LBC also resulted in increased laboratory productivity and efficiency, with no adverse effect on quality. A large laboratory in Manchester reported that nearly 1 min per slide was saved during primary microscopy, and microscopy by cytopathologists, using LBC compared with conventional smear preparations. The uninterrupted hourly rate of slide examination rose from 8.6 slides for conventional smear preparations to 11.7 for LBC preparations, comparable to the data from the Scottish LBC feasibility study [76, 85]. A separate study from Scotland reported a 40% reduction in full primary screening time [86]. In the Sheffield laboratory, individual screener productivity increased by 20% in the first year following full LBC implementation [87], and productivity increases of up to 50% coupled with decreased numbers of unsatisfactory samples and an increased sensitivity for the detection of cytological abnormalities validated by subsequent histological investigation have been reported [88].
Increased productivity was also reflected in national data showing a progressive increase in the proportion of laboratories reporting results within 2 weeks of specimen receipt, an important achievement in view of the Cancer Reform Strategy objective that all women should receive the results of their test within 2 weeks by 2010 [89, 90].
As a result of LBC implementation, there was a growing mismatch between workload and capacity in some laboratories. However, a NHSCSP workforce survey revealed that over one-third of screening staff were over 50 years of age, and LBC implementation buffered laboratories against this marked demographic change [91]. In fact, some laboratories found no need to replace primary screening staff on retirement, resulting in cash-releasing cost savings.
National implementation of LBC not only resulted in improved laboratory efficiency and productivity but was also the platform for consideration of the implementation of molecular testing and automation in the NHS cervical screening programs.
HPV Testing
The recognition of the strong causal relationship between persistent infection of the genital tract with high-risk human papillomavirus (HPV) types and the occurrence of cervical cancer has resulted in the development of a number of HPV DNA and RNA detection systems in an attempt to refine existing cytology-based cervical cancer screening programs [92–95] (see Chap. 2). LBC provides an ideal platform for application of this and other molecular technologies. Detection of high-risk HPV DNA is considered to be potentially useful in four clinical applications [96]:
- 1.
As a triage test to select which women who have low-grade cytological abnormalities in routine screening require immediate referral for colposcopy rather than cytological surveillance.
- 2.
Follow-up of women with abnormal screening results who are negative at colposcopy and biopsy.
- 3.
Follow-up for women treated for high-grade CIN with local ablative or excisional treatment to more rapidly and accurately identify those who have or have not been cured.
- 4.
As a primary screening test, either alone or in combination with cervical cytology to detect cervical cancer precursors.
Triage of Low-Grade Abnormality
A meta-analysis of studies published between 1992 and 2010 comparing HPV testing with Hybrid Capture 2 (HC2) with repeat cytology in the management of low-grade cytological abnormality (borderline nuclear change/atypical squamous cells (ASCUS); mild dyskaryosis/low-grade squamous intraepithelial lesion (LSIL)) showed that HPV triage with HC2 of women with borderline nuclear change had significantly higher sensitivity than, and similar specificity to, repeat cytology. In triage of women with mild dyskaryosis, an HC2 test yielded a significantly higher sensitivity, but a significantly lower specificity, compared to repeat cytology [97]. A pilot study conducted within the initial English evaluation of liquid-based cytology demonstrated that, while HPV triage of low-grade abnormality resulted in a reduction in the rate of repeat smears but an increase in rates of referral to colposcopy, it was likely to be cost effective [98, 99]. A further evaluation of HPV triage implementation in six laboratories in the English cervical screening program (the sentinel site study) demonstrated that triaging women with low-grade cytological abnormalities by HPV testing would allow approximately a third of these women to be returned immediately to routine recall, and immediate referral for colposcopy would avoid the need for repeat cytology in the remainder. The HPV-positive rates at the six sites ranged from 34.8% to 73.3% for women with borderline cytology and from 73.4% to 91.6% for women with mild dyskaryosis, and these differences remained after the rates were standardized for age. Overall the HPV-positive rate was higher in sites using ThinPrep® than in those using SurePath® LBC [68.7% and 61.7% respectively (p < 0.001)], and the difference remained after adjustment for age group and initial cytology result. LBC technology was, however, confounded by site, and it was therefore not possible to determine whether this difference was due to variation in the reporting of cytology between sites. In the only site which used both technologies, there was no significant difference in positive rates between the two technologies [100]. Based on this data HPV triage of low-grade cytological abnormality was implemented in the English cervical screening program in 2011 using the algorithm developed for the sentinel site study (Fig. 3.2).
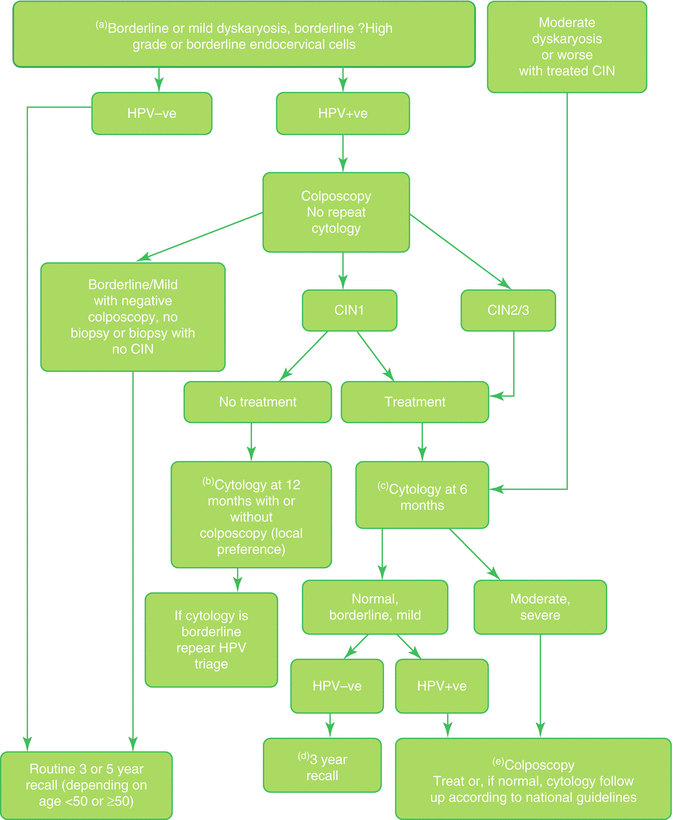

Fig. 3.2
Flow chart: triage and test of cure in the NHSCSP (© Crown Copyright 2016). This information was originally developed by Public Health England Screening (https://www.gov.uk/topic/population-screening-programmes) and is used under the Open Government Licence v3.0
Test of Cure
Prior to 2011 NHSCSP guidance was that women treated for low-grade disease (CIN 1) required follow-up cytology at 6, 12, and 24 months and if all results were negative could return to routine screening. Women treated for high-grade disease (CIN 2 or 3 or cervical glandular intraepithelial neoplasia (CGIN)) required 6- and 12-month follow-up cytology and annual cytology for the subsequent 9 years at least before returning to screening at the routine interval. It has been estimated that in England every year more than 300,000 cytology tests were performed annually for follow-up after treatment, approximately 10% of the annual workload [101]. A number of studies prior to 2007 demonstrated that testing for high-risk HPV infection with Hybrid Capture 2 was more sensitive, though less specific, than repeat cytology in the detection of residual disease following excisional treatment of high-grade CIN, and a large prospective study from the UK showed that a negative result from a high-risk HPV test after treatment was indicative of very low risk of recurrent disease even in the presence of low-grade cytological abnormality: women who were cytology and HPV negative at 6 months could safely be returned to routine three-yearly recall [102, 103]. Evaluation of HPV as test of cure after treatment of CIN in the sentinel study demonstrated that about 85% of treated women were HPV negative at 6 months after treatment [100]. HPV test of cure was implemented with HPV triage of low-grade cytological abnormality in the English cervical screening program in 2011 (Fig. 3.2).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


