Fig. 16.1
Encephalocele. a Cut section of encephalocele wall showing skin surface with dermis and epidermis a dense fibrous dura-like band and deep-seated leptomeninges. b Brain tissue covered by a layer of leptomeninges. c Leptomeningeal tissue with reactive glial cells (arrows) and short curvilinear bands of collagen. d Malformed brain tissue. Neurons are seen in the inset
Frontoethmoidal encephaloceles |
Nasofrontal |
Nasoethmoidal |
Nasoorbital |
Cranial vault encephaloceles |
Interfrontal |
Anterior fontanel |
Interparietal |
Posterior fontanel |
Temporal |
Cranial base encephaloceles |
Transethmoidal |
Sphenoethmoidal |
Transsphenoidal |
Frontosphenoidal |
Occipital encephaloceles |
Cranioschisis |
Associated with cranial/upper face clefts |
Associated with basal/lower face clefts |
Occipitocervical clefts |
Acrania and/or anencephaly |
Biology and Epidemiology
No known genetic mutation.
Higher incidence in Southeast Asia (1:6,000 live births) than North America (1:35,000 live births) [2].
Theories of Pathogenesis
Embryology
Neural tube begins to close between 3rd and 4th week of fetal development.
Neural crest cells migrate into the frontonasal and maxillary processes, differentiating into the facial bones, cartilage, and muscles.
Abnormal development of the potential spaces between these developing structures (fonticulus frontalis, prenasal space, foramen cecum) is responsible for congenital midline masses.
Presentation
Soft, compressible, pulsatile midline mass that transilluminates
Occasionally may present with ulcerations and leaking CSF, which requires emergent closure
Differential Diagnosis
The three most common diagnoses of a midline mass in an infant are dermoid cyst, glioma, and encephalocele [6, 7]. History and physical exam can generally lead to the correct diagnosis; however, this is usually confirmed with imaging.
Nasal dermoid cyst
Most common midline mass
Present at birth, diagnosed in early childhood
Composed of ectodermal and mesodermal elements
Hallmark is punctum with a single hair located on the nasal dorsum
Often become infected and can drain sebaceous material
Intracranial extension cannot be ruled out on exam—imaging required for accurate diagnosis
Nasal glioma
Presents as firm rubbery mass with bluish or reddish appearance
Composed of glial cells in a connective tissue matrix
Often extend intranasally
Does not communicate with cerebral contents, so not pulsatile and does not transilluminate
Less common entities that occur in the midline
Vascular malformation
Teratoma
Sebaceous cyst
Neurofibroma
Ganglioneuroma
Nasal fibroma
Adenoma
Chondroma
Carcinoma
Diagnosis and Evaluation
Patients who present with an encephalocele are most appropriately managed by a multidisciplinary craniofacial team comprised of a craniofacial surgeon, a neurosurgeon, an otolaryngologist, a geneticist, and a pediatrician. Neurological and developmental assessments and evaluation by an ophthalmologist are also essential.
Physical Examination
Soft nasal mass in midline is the most common presentation
Bluish appearance
Soft, compressible, and pulsatile mass that transilluminates
Mass increases with size with crying, Valsalva, or compression of internal jugular veins (Furstenberg test)
“Long nose hypertelorism”—patients present with long, flat, wide noses that is more pronounced after encephalocele excision [8, 9]
True orbital hypertelorism is rare, but telecanthus and interorbital hypertelorism are universal
Deformational trigonocephaly
Laboratory Data
No specific laboratory test confirms the diagnosis of encephalocele. A preoperative hemoglobin is prudent.
Imaging Evaluation
Computed tomography (CT) scan is the imaging modality of choice.
Analyzing both bone and brain windows in the axial, coronal, and sagittal planes, as well as three-dimensional reconstructions, are necessary for understanding the complex bony and intracranial anatomy involved.
Useful for assessing the potential presence of hydrocephalus.
Sagittal reconstructions helpful for evaluating the presence of Chiari I malformation, relevant to patients at risk for hydrocephalus (can also be seen on magnetic resonance imaging, MRI).
Essential for planning a successful operative intervention.
MRI.
Provides the most detailed soft tissue images and is useful in distinguishing between soft tissue masses.
Ultrasound.
May be useful in evaluating for hydrocephalus, but often redundant if CT or MRI performed in initial evaluation.
Pathology
Diagnosis can be made with a combination of history, physical exam, and imaging. Biopsies prior to definitive repair are unnecessary and should be discouraged. Histopathologically, meningoceles consist of leptomeningeal membranes with or without glial tissue and meningoencephaloceles of malformed brain tissue and the leptomeningeal membranes covering it (Fig. 16.1). Ependymal tissue may be be seen in hydroencephalomeningoceles.
Treatment
Medical
No medical intervention exists to treat this anatomical abnormality.
Surgical
Operative intervention provides definitive correction of this problem (see Fig. 16.2). Successful correction follows the following principles [10]:
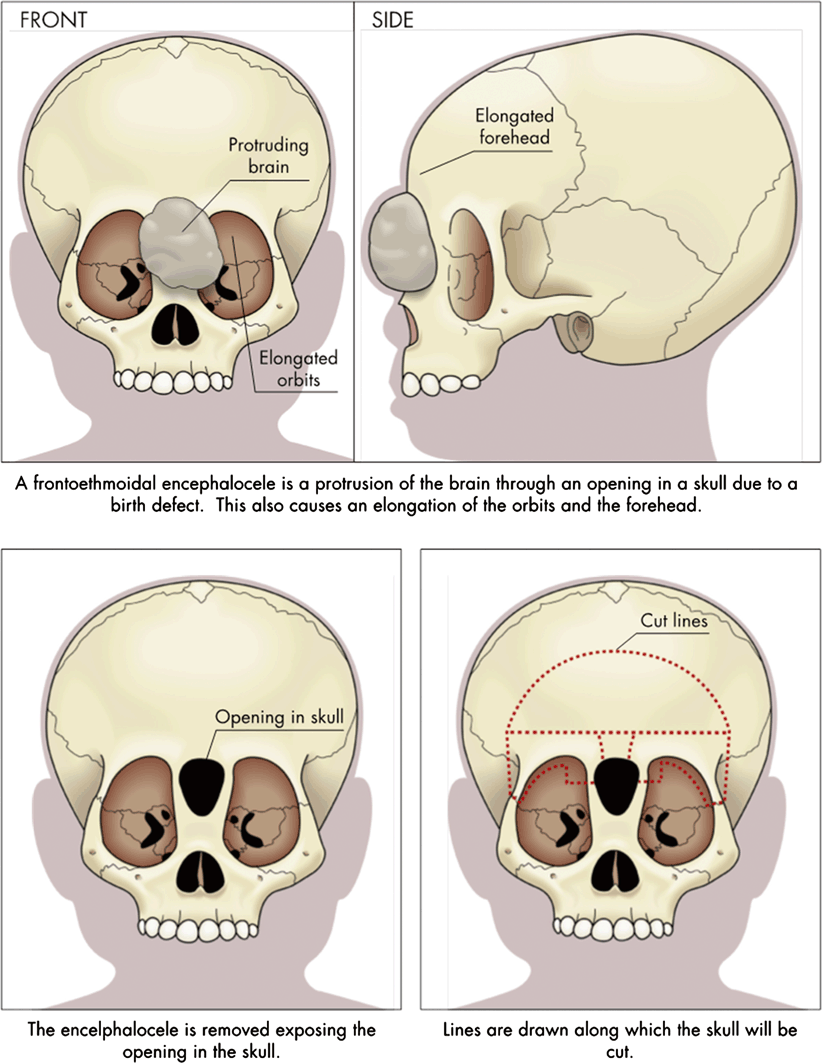
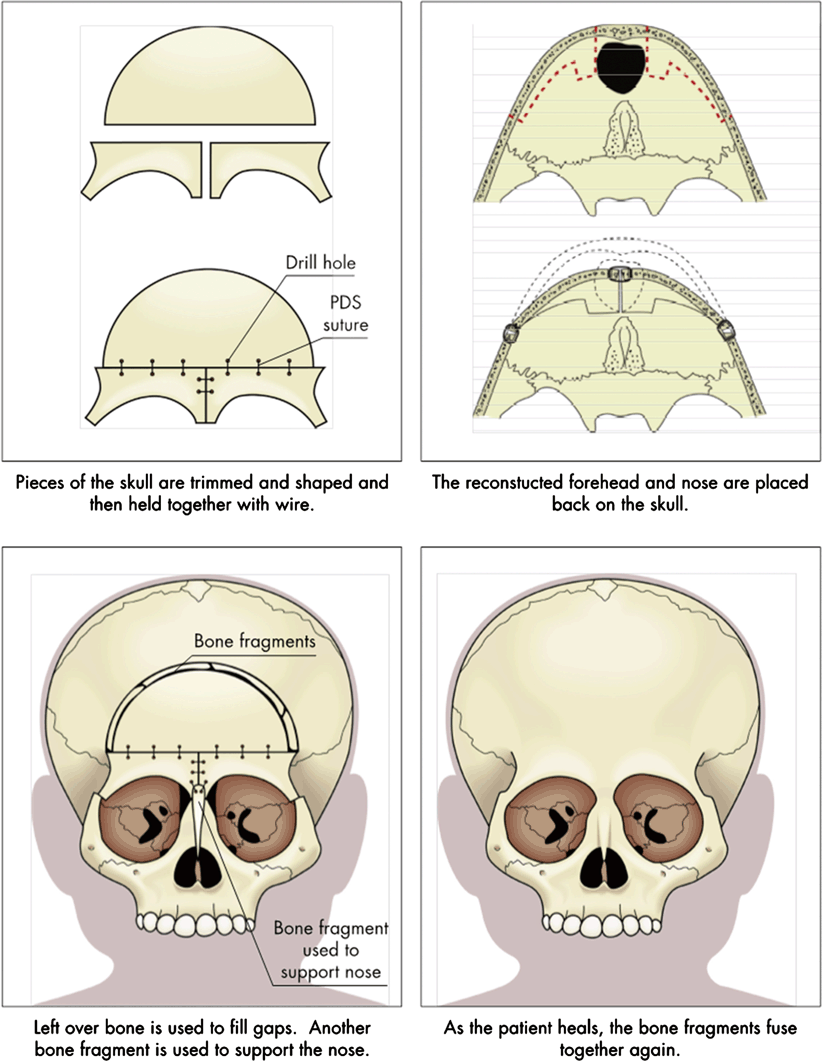
Fig. 16.2
Reconstruction of frontoethmoidal encephaloceles
Accurate diagnosis, delineation of anatomy, and surgical planning
Single-stage operation with both craniofacial surgeon and neurosurgeon present
Osteotomies and bone movements that correct all deformities, including the trigonocephalic head shape and interorbital hypertelorism
Nasal reconstruction that avoids the long-nose hypertelorism deformity
Skin closure that removes abnormal skin and places incisions in advantageous locations
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


