Artificial cycle – FET. Luteal Lupron (GnRH agonist) and OCP –> Lupron start illustrated
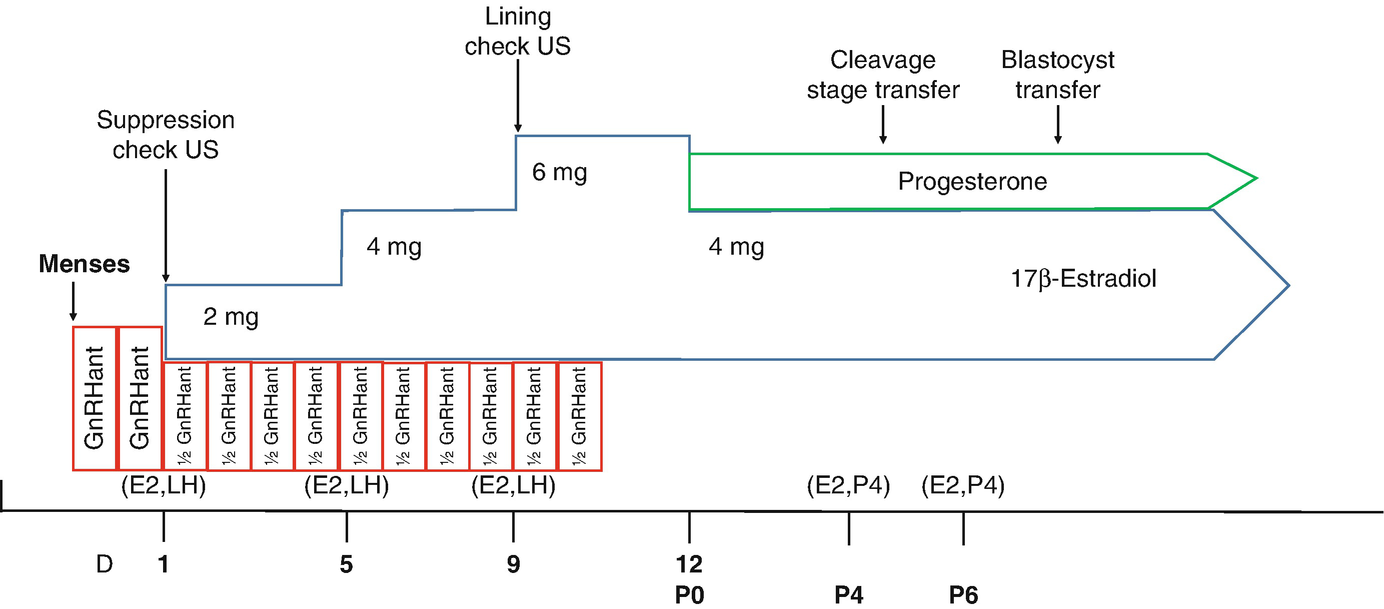
Artificial cycle – FET. GnRH Antagonist Cycle Protocol illustrated
21.2.2 Endometrial Lining (Stripe)
In addition to carefully monitoring the progressive rise of E2 levels during the FET cycle, the most important checkpoint prior to starting progesterone is the assessment of the endometrial lining appearance and sonographic thickness. Classically, a thin endometrial lining has been defined as both <7 mm and <8 mm in frozen-thaw embryo transfer cycles [24–29]. The reported incidence of thin lining in assisted reproductive technology (ART) cycles ranges between 1.5% and 9.1% [24, 26–29]. Theories regarding the impact of thin endometrium and poor ART outcomes involve possible poor growth of the endometrial glandular epithelium, decreased vascular endothelial growth factor (VEGF), poor vascular development, higher uterine blood flow impedance, and the oxidative effects of close vascular supply [30].
Select studies have looked at the impact of endometrial lining thickness specifically for FET. Findings of one such study found that the lowest pregnancy rates were associated with endometrial lining thickness of <7 mm and >14 mm and significantly higher rates of clinical pregnancy and subsequent live birth were achieved with endometrial thickness in the 9–14 mm range [25]. One of the largest studies to date examined whether each millimeter of decreasing endometrial thickness resulted in decreased pregnancy and live birth rates. This analysis of the large Canadian ART Registry (CARTR-BORN database) included a total of 20,114 frozen-thawed embryo transfer cycles from the time period of January 1, 2013, to December 31, 2015 [31]. Their findings showed that for FET cycles, both clinical pregnancy and live birth rates decreased with each millimeter below 7 mm, with no significant difference in miscarriage rates for each decreased millimeter. Most interesting in this analysis was the findings of diminishing but not markedly decreased pregnancy rates for even very low endometrial thickness levels. The study found live birth rates of 28.4, 27.4, 23.7, 15, and 21.2% and for endometrial thickness ≥8 mm, 7–7.9 mm, 6–6.9 mm, 5–5.9 mm, and 4–4.9 mm, respectively [31]. The slight increase in live birth rates for the 4–4.9 mm group likely represents a statistical artifact due to small number of transfers in this group, but nonetheless it does illustrate that even with decrease in endometrial thickness all the way down to <5 mm, pregnancy is possible.This study can be cited as a valuable counseling tool in patients with persistently thin endometrium who insist on autologous transfer.
Thus it can be surmised from the available data that a lining thickness >8 mm is ideal and that >7 mm is acceptable. Review of lining morphology and thickness during ovarian stimulation may reveal that supraphysiologic levels of E2 are needed to achieve a sufficient endometrial lining; thus dosing of E2 can be increased or extended if needed. However, care must be taken with this as markedly elevated levels of E2 (E2 levels >700 pg/mL) were implicated in decreased live birth rates [32]. Despite adequate E2 levels and adequate duration of E2 exposure, an endometrial lining that is persistently <7 mm is sometimes encountered and can be a clinically challenging situation. Options for these patients include what could be called “experimental” adjuvant treatments. Protocols such as intrauterine infusion of granulocyte-stimulating factor (G-CSF), co-treatment with sildenafil (Viagra™, Pfizer), and infusion of autologous platelet-rich plasma have been used in the setting of refractory thin endometrium [33–37]. There have even been pilot studies using bone marrow-derived stem cells (BMDSCs) in attempts to improve endometrial thickness in patients with refractory thin endometrial lining [38]. These approaches have yet to be validated in large well-designed studies.
In patients with persistently thin endometrium, issues such as prior surgical history causing Asherman’s syndrome or chronic endometritis must be ruled out as potential causes of this phenomenon and corrected where possible. Specifically with chronic endometritis, improvements in implantation rate have been observed following treatment in patients with recurrent implantation failure [39]. Hysteroscopic lysis of adhesions may be indicated if intrauterine synechiae are suspected.
In addition to the endometrial thickness, the morphology and the characteristics of the endometrial lining merit close attention. Various publications have defined the phases of the endometrium in different ways. For the purposes of this chapter, the following nomenclature will be used: pattern 1 (late proliferative: hyperechoic endometrium measuring <50% of the endometrial thickness with a hypoechoic functionalis and a hyperechoic basalis), 2 (early secretory: hyperechoic basalis and functionalis extending >50% of the endometrial thickness, but not encompassing the entire endometrial cavity), and 3 (mid-late secretory: homogeneous hyperechoic functionalis extending all the way from the basalis to the lumen) [40]. Pattern 1 is considered the characteristic “trilaminar pattern” that is the hallmark of the mature proliferative endometrium. Overall, type 1 endometrial pattern (“trilaminar”) is associated with improved pregnancy rates [41–44]. All these studies used embryo morphology as the criteria for embryo selection. A more recent study using preimplantation genetic testing for aneuploidy (PGT-A) confirmed euploid embryos (in theory removing to some extent chromosomal factors with the embryo) and found no statistically significant difference in implantation rate between pattern 1 and pattern 2 linings but showed decreased implantation rates for pattern 3 linings [45]. Given this, our clinical goal is the creation of a pattern 1 endometrial lining with at least 7 mm of thickness.
Lastly, during the endometrial lining assessment prior to progesterone administration, careful attention is also paid to the dynamic character of the endometrial stripe itself. Additionally, the presence and the extent of sub-endometrial contractions on 2D ultrasound are also carefully noted. Uterine contractility is a normal phenomenon during the menstrual cycle, reaching its peak in the late follicular/peri-ovulatory phase before subsiding greatly in the luteal phase [46]. Different studies assessed uterine contractility at various points in the cycle: the day of trigger in a fresh embryo transfer cycle [47], just prior to embryo transfer [48, 49], and at the time of embryo transfer [50, 51]. This has led to mixed results with some studies finding no correlation between sub-endometrial contractions and clinical outcomes [49], while others showed decreased pregnancy rates in those with the highest level of contractions [48, 50, 51]. In our practice, no ultrasonographic assessment of the lining is done after progesterone administration. If severe sub-endometrial contractions are seen at the time of lining assessment (contractions that resemble in character and speed the peristalsis of small bowel), then strong consideration is given toward cancelling that cycle. Anecdotally, such severe contractions are noted in patients who have had markedly fluctuating E2 levels during the endometrial preparation phase of their FET cycle, especially in cases that have had drastic falls in their serum E2 levels. If this was the case, alternative dosing strategies are sought in subsequent cycles to minimize changes in E2 levels.
21.2.3 Progesterone Supplementation/Luteal Phase Support
Similar to estrogen supplementation, the optimal progesterone supplement for luteal phase support in FET cycles has yet to be agreed upon. Various dosage forms have been developed and each has their own particular characteristics and potential drawbacks. Furthermore, owing to the heterogeneity between different approaches to FET, it can be hard to extrapolate from one study to another. Additionally, data from luteal phase support in IVF cycle cannot be extrapolated to artificial FET cycles as there is no formed or functioning corpus luteum; thus, all the progesterone needed to transform the endometrium and to maintain the early pregnancy is coming from an exogenous source.
Unfortunately, few studies directly examining luteal phase support in AC-FET cycles are available. Two small, randomized, prospective trials comparing vaginal progesterone with IM progesterone in donor oocyte recipients showed no difference in terms of ongoing pregnancy rates [52, 53]. Other retrospective studies examining donor oocyte recipients [54] and a study looking at both recipients of donor and autologous frozen blastocysts [55] also showed no differences in implantation, clinical pregnancy, or live birth rates. In contrast, two other showed decreased live birth rates in patients receiving vaginal progesterone (22.8% vs 34.5%) [56] and (24.4% vs 39.1%) [57]. Thus the best available data is not entirely clear on which form is superior.
A few issues should be discussed in regard to the benefits of IM progesterone despite some clear drawback. As discussed previously, estrogen is known to increase uterine contractility and progesterone antagonizes this action, thus reducing the extent of sub-endometrial contraction activity. Progesterone, when given vaginally, achieves higher endometrial concentrations then intramuscular progesterone despite lower serum level [58]. While vaginal progesterone does mature the endometrium at a faster rate than IM progesterone, the short half-life of vaginal administration necessitates more frequent dosing. It is possible that the intermittent peaks and troughs of progesterone result in less uterine quiescence than the progesterone in oil depot effect giving longer sustained tissue levels that promote a more evenly “relaxed” uterine environment that may give rise to higher implantation rates. Additionally long gaps in time do not occur with IM administration as they do with the overnight gap between the evening and morning vaginal progesterone administrations. When assessing the ability to reduce sub-endometrial contractility, a small randomized trial (n = 34) showed no difference between vaginal and IM progesterone in reducing endometrial contractility at the time of ET so this matter is still up for the debate [51]. A 2010 Cochrane review, with a total of four trials satisfying criteria for analysis, found no statistically significant difference in regard to live birth, clinical pregnancy, or miscarriage rates between vaginal and IM progesterone [59]. Still the authors acknowledge that further study concerning the optimal route of progesterone delivery is still needed. One recent trial that is worth mentioning examined the use of vaginal-only progesterone (Endometrin 200 mg BID; Ferring Pharmaceuticals), vaginal progesterone (Endometrin), and IM progestin every 3rd day or daily IM progesterone. The results of their interim analysis concluded that relative to IM progesterone and combination vaginal-IM progesterone, vaginal-only progesterone resulted in a decreased ongoing pregnancy rate due to a higher miscarriage rate in the vaginal-only treatment group [60]. The biggest takeaway from this study may be the equivalent outcome of combination therapy of progesterone which may allow some mitigation of the side effects of vaginal and IM administration. Further study is still needed to validate this approach.
Nonetheless, both IM and vaginal progesterone have their drawbacks when it comes to patient experience and patient satisfaction. IM progesterone can be painful, especially for long durations of use, and can cause sterile intramuscular abscesses. Vaginal progesterone may cause vaginal irritation in some women. The drawbacks of these methods have led some to explore oral dosing of progesterone to achieve the same ART success as other methods while limiting the side effects that are common with the other dosage forms. Oral preparations of progesterone have generally been avoided due to lower bioavailability and worse ART outcomes [61–63]. That said, dydrogesterone, a retroprogesterone with excellent oral bioavailability [64], currently not available in the United States, has been well researched in regard to luteal phase support after fresh cycle IVF and has shown equivalent outcomes [65–69]. Unfortunately, only limited data exists in its use for FET cycles. Two small studies examined the oral dydrogesterone as the sole source of progesterone in a FET cycle with one study finding comparable results to vaginal progesterone [70], while the other study reported lower pregnancy rates [71]. As with many issues concerning frozen cycles, further study is needed to define the feasibility of an oral-only approach, and if so, what is the optimal dose needed to achieve at least equivalent outcomes to more tried and true methods.
21.3 Natural Cycle
Next, we move on to the so-called “natural cycle” FET approaches. As with minimal/mild stimulation and ovarian stimulation approaches, no consistent definition of what exactly constitutes “natural” is agreed upon. The International Society for Mild Approaches in Assisted Reproduction (ISMAAR) proposed the following definition as it relates to IVF cycles: natural cycle IVF connotes unstimulated, spontaneous IVF cycles; modified natural cycle IVF connotes “semi-natural,” controlled natural cycle IVF with hCG-only antagonist and FSH or HMG add-back [72]. We will extend the use of these terms to FET. Therefore, natural cycle FET (NC-FET) denotes no exogenous medications used during the endometrial growth phase and the corpus luteum is the sole source or progesterone. In modified natural cycle (mNC-FET), estrogen, progesterone, and even hCG can be added to support the underlying natural physiologic process.
The benefits of natural/modified natural cycle FET are numerous. These include using a more physiologic means of preparing the endometrium for implantation. This means little to no medication is needed resulting in less medication cost to the patient. Additionally, avoiding the time and medication needed to suppress the hypothalamic pituitary axis via OCPs and GnRH agonist once again reduces medication cost and side effects and allows a shorter time to begin the FET cycle. Finally, as some patients express discomfort with both vaginal and IM administration of progesterone, avoiding luteal phase support can increase patient satisfaction as well as reduce cost. Unfortunately, natural/modified natural cycle techniques are not suited to patients with irregular cycles. This can especially be an issue for patients with ARA and/or DOR as they are prone to suffer the menstrual cycle characteristics typical of diminishing ovarian reserve, namely, short follicular phases, erratic follicular development (with accompanying erratic E2 levels), impaired corpus luteum development/function, and luteal phase insufficiency. To ensure these conditions are not present, intense laboratory monitoring with often daily lab draws is needed around the time of ovulation, and thus patients with limited access or unwillingness to attend intense monitoring would not be good candidates for this approach. The heightened demands of natural approaches may also burden the fertility clinic schedules as well. The unpredictability of cycle starts and timing of the FET can be stressful from a planning and staffing standpoint as the day of transfer can often fall on a weekend and occasionally on holidays.
A randomized controlled, non-inferiority trial (ANTARCTICA trial) compared artificial cycle with modified natural cycle. The study was conducted between February 2009 and April 2014 and it included an analysis of a total of 959 (n = 959) cycles and looked at 495 modified natural cycles and 464 artificial cycles. Using live birth as the primary outcome, modified natural cycle was shown to be non-inferior to artificial (LBR 11.5% for mNC-FET vs 8.8% AC-FET, 2.7% in favor of mNC-FET (95% confidence interval (CI) −0.065–0.012; P = 0.171) [23]. Similarly, there was no significant difference in clinical pregnancy rate or ongoing pregnancy rate observed. Interestingly, the cancellation rate for AC-FET was significantly higher than for mNC-FET (26.7% vs 20.4%, OR 1.4 95% CI 1.1–1.9 P value 0.02).
One question encountered during clinical practice is whether there needs to be a delay or an interval of time between the ovarian stimulation and the FET. Several retrospective studies examined this issue and have shown no difference between a delay of treatment and immediate start of post-ovarian stimulation and oocyte retrieval [73–76]; thus FET preparation can begin without delay with the first menses following oocyte retrieval.
21.3.1 Monitoring During NC
Integral to the success of a NC-FET is careful monitoring of the hormonal and ultrasonographic parameters as the cycle progresses. While a strict protocol for natural cycles is a contradiction in terms (i.e., a natural cycle varies for each patient and cannot necessarily be made to follow a strict set of rules), at our institution, we generally have a few principles which guide our efforts.
In a patient with regular menstrual cycles, they are instructed to contact the clinic with the onset of full flow of menstrual bleeding. This is considered cycle day 1 (CD1). They are then brought back on cycle day 10 for estradiol (E2), luteinizing hormone (LH), and progesterone (P4) levels. Based on the early values, they are then brought back for only blood work on a daily or every other day basis until their E2 level reaches ~200 pg/mL. At this time, they are brought in for ultrasound to assess the growth of the dominant follicle and the endometrial lining thickness. The goal lining thickness is at least 7 mm with trilaminar morphology. If the desired lining thickness is not achieved on the initial ultrasound, they can be brought back in the coming days as long as the E2 level is still rising and the P4 level is <1 ng/mL. Once the endometrial lining is at least 7 mm in thickness, the patient will come in for daily blood work to confirm ovulation (LH surge >15 IU/L, drop of E2 concentration, a rise in serum progesterone level). Some centers confirm collapse of the known follicle, but this is something we do not routinely do unless indicated by abnormalities in the hormone levels. As the progesterone rises to >1.5 ng/mL, it is our practice to begin progesterone in oil (PIO 50 mg/mL) at a dose of 1 mL IM injection. Additionally, micronized estradiol 2 mg (Estrace®, Teva Pharmaceuticals, Sellersville, PA) is started as well. Both of these medications function to “support” the underlying physiologic process. At the initiation of estrogen and progesterone, adjuvant medications like antibiotics and corticosteroids are also begun. As far as timing of the transfer, closely following the LH levels allows us to see the day of LH surge and thus we can calculate the date of the theoretical oocyte retrieval/pick up (tOPU). From this, we then transfer at the cleavage stage or blastocyst stage depending on the patient’s frozen embryo status.
21.3.2 Modified Natural Cycle
To counter some of the unpredictability as well as to augment or shore up any lacking hormonal parameters, some centers have explored adding in various preparation to either support or augment the underlying physiologic processes that make up NC-FETs. This has given rise to the modified natural cycles (mNC-FET). One strategy explored has been the addition of an HCG trigger to the cycle rather than relying on spontaneous ovulation. Proponents of this technique cite several benefits to its use. Firstly, it allows some control and flexibility over the timing of ovulation so that there is some element of control over the timing of embryo warming and transfer. Secondly, it may be possible to decrease the number of monitoring/lab visits as spontaneous ovulation is not being awaited passively [77, 78]. Lastly, the addition of HCG may play a “support” role toward the corpus luteum thus potentially treating possible luteal dysfunction if it exists [79]. Negatives would include the increased cost of the medication and discomfort/inconvenience associated with injections and potential negative impacts on the endometrium which will be discussed below [80, 81].
One early prospective randomized controlled trial from 2010 directly compared outcomes for FET between spontaneous ovulation and induced ovulation via HCG trigger. This study notably did not give luteal support and an LH surge detected at the time HCG trigger was not an exclusion criteria. These factors may explain why this study was stopped prematurely as they noted a substantial decrease in ongoing pregnancy rate in the HCG group vs the pure natural cycle (14.3% vs 31.3%; p = 0.025) [80]. Another study by Montagut et al. also found the addition of HCG trigger to natural cycle decreased the pregnancy rates [81]. The authors of these studies suggested a possible negative impact of HCG on the endometrium via alterations of the luteinizing hormone/choriogonadotropin receptor (LHCGR) localization after exposure to exogenous HCG relative to changes that occur via endogenous LH exposure [80, 81].
In contrasts, two studies by Weissman et al. also compared the clinical outcome of pure NC-FET versus the addition of HCG trigger. The criteria for administration of HCG was the following: (i) existence of a dominant follicle at least 17 mm visualized by transvaginal ultrasound, (ii) serum progesterone <1 ng/mL, and (iii) serum E2 >150 pg/mL. An endometrial thickness of at least 7 mm was required prior to moving forward with the embryo transfer. As noted previously, luteal support was given in these studies. Both studies showed no statistically significant difference in implantation rate, clinical pregnancy, or live birth rate per transfer. Only the reduced number of monitoring visits was statistically significant in favor of the HCG trigger group [77, 78].
An important issue to consider regarding the use of HCG trigger relates to the interruption of the careful feedback between the developing follicle, the pituitary, and the developing endometrium. As has been noted many times previously [82], ovulation does not occur at a fixed dominant follicle size nor an exact E2 level. Allowing the body to decide on the optimal ovulatory time may maximize endometrial development and perhaps increase receptivity.
Lastly, the need for luteal phase support (LPS) has been questioned in regard to NC-FET. There have been a few studies published regarding this matter, and the results have been conflicting. In a retrospective analysis, Kyrou et al. showed no benefit for the inclusion of LPS with mNC-FET, while in contrast Bjuresten et al. showed higher live birth rates when LPS was added to true natural cycles [83, 84]. Still, others showed no difference in NC-FET with addition of LPS [85]. Furthermore, the exact route and dosage of LPS has not been universally agreed upon adding further confusion. Given these discrepancies, at this time there is a paucity of evidence in regard to recommending luteal phase support in NC-FET; thus this may be left up to provider/clinic preferences. As previously mentioned, our standard practice is to add low-dose estrogen and progesterone to our natural cycle patients once ovulation has been confirmed especially in women with DOR and ARA.
21.4 Additional Medications
In this next section, we will discuss some of the other medications that are sometimes used during an FET cycle apart from the main hormonal medications previously described in this chapter. Additionally, we will highlight the additional medications used in FET cycles performed at our center.
At the time of embryo transfer (ET), a catheter is placed through the cervical os to access the uterine cavity. Due to theoretical concerns of tracking upper genital tract microbes from the vagina into the uterus via the catheter, antibiotic administration prior to ET has been proposed as a way of improving pregnancy rates. Cochrane database analysis of this issue found that the use of amoxicillin and clavulanic acid prior ET reduced upper genital tract microbial contamination but did not alter clinical pregnancy rates [86]. This same finding was supported in another study [87], and thus according to most sources, the routine use of antibiotics prior to ET is not indicated [86, 88]. That said, it is our practice that all patients (fresh and frozen) receive 3 days of antibiotics (azithromycin 250 mg daily for 3 days) initiated at the start of progesterone initiation in fresh and frozen cycles.
Another medication often used as an adjuvant in ART is aspirin. Aspirin has been employed during FET cycles due to the potential for enhanced uterine perfusion and thus enhanced endometrial receptivity [89, 90]. The use of aspirin has shown mixed results when used in fresh transfer IVF cycles [90–93]. Data looking exclusively at frozen cycle outcomes is more limited. One early study found improved pregnancy rates by using high doses of aspirin 150–300 mg/day [94], but later small study found no improvements in pregnancy rate [95]. However, a more recent larger pilot randomized, double-blind placebo-controlled trial showed significant improvements in implantation rate, clinical pregnancy rate, and most importantly live birth with daily low-dose aspirin [96]. Importantly, this study used the standard and readily available dosing of 81 mg (“baby aspirin”). Given this, it is our standard practice to include aspirin in all of our frozen -thaw cycles (both natural and medicated). We continue baby aspirin through the first pregnancy test and subsequent ultrasounds. We do have a low threshold for stopping aspirin if any first trimester bleeding occurs.
Lastly, we will address the use of peri-implantation corticosteroids to embryo implantation. The theory behind this intervention is that the use of steroids will modulate uterine natural killer (NK) cells, improve the cytokine/growth factor milieu in the endometrium, and suppress endometrial inflammations in the hopes of improving receptivity [97, 98]. The most recent Cochrane review of 14 trials found no effect on clinical pregnancy or live birth rate in the general IVF/ICSI fresh transfer population [97]. Two nonrandomized trials revealed a benefit in patients with recurrent implantation failure [99, 100]. A large randomized controlled trial involving the use of corticosteroids alone or in combination with aspirin in frozen transfer cycles is lacking at this time. Furthermore, a trial examining the use of corticosteroids in cleavage state transfer in patients with advanced reproductive age and diminished ovarian reserve (reflective of our practice’s patient population) needs to be performed. Given this, we prescribe 16 mg of methylprednisolone for 3 days starting at the time of progesterone initiation.
21.5 Adjunct Procedures
If an adequate trilaminar lining is achieved and there are no pregnancy results after the transfer of a high-grade embryo, one of the thoughts/suspicions is the failure of synchronization between the endometrium and the embryo resulting in failure to implant. Couples with an unsuccessful embryo transfer may feel desperate and inquire as to additional procedures or interventions that could be performed to increase their chances in subsequent cycles. Two of the most commonly asked for adjunct procedures among our patients are endometrial receptivity testing and endometrial scratch.
During the menstrual cycle, there is thought to be a “window of implantation” when the endometrium is receptive to the embryo [79]. One of the theories behind the lack of implantation of a “high-quality” embryo is since there is a mismatch between the “timing” of the endometrium and the “timing” of the embryo. By performing a mock cycle and obtaining a biopsy, an endometrial receptivity array (ERA) can be performed to guide the physician with transferring the embryo in a subsequent cycle. Our center has yet to adopt this in our patients. One of the central assumptions of ERA testing is that by repeating the same medication protocol, the same endometrial development and biopsy findings should always be the same over repetitive cycles. Therefore the transfer day should be indicated by the results of the ERA test as determined by the prior mock cycle. However, in our close observation of medicated cycles, we often observe quite dramatic fluctuations in serum levels of estradiol regardless of the dosage form (hence our strategy of frequent lab testing). Just as we feel there are variations from cycle to cycle in terms of ovarian response, we similarly feel there is cycle-to-cycle variation in endometrial preparation. As more research become available in the future, this issue may be revisited.
Local endometrial injury results in an inflammatory response that favors implantation. Specifically, this intervention was sought out by patients with recurrent implantation failure (RIF) as means of improving their chances of having a successful outcome. Some evidence exists in smaller studies; thus on occasion we have performed this upon request from patients (usually at the time of cavity assessment prior to an FET cycle). Additionally, by performing the endometrial scratch via an endometrial biopsy pipelle, a sample of tissue can be sent to test for chronic endometritis which has been shown to be more prevalent among patients with RIF. However, as a result of a recent large, well-designed, randomized controlled trial, there will likely be a decreased use of this technique in the future. The Pipelle for Pregnancy (PIP) trial investigated whether endometrial scratch via endometrial biopsy increased the probability of live birth. Endometrial scratching did not result in higher rates of live births in patients undergoing either their initial transfer or in those who had previously failed transfer [101]. It is interesting to note that this large study did not observe benefit in those with previous implantation failure given that this is the exact population that was purported to benefit the most from endometrial scratch.
Lastly, we can briefly mention that all our embryo transfers are performed under pelvic ultrasound guidance by using Wallace® Sure-Pro® embryo replacement with obturator (Cooper Surgical, Trumbull, CT). The cervix is gently irrigated by using 10 mL of sterile media to minimize mucus and debris. After passing the cervical internal os with the outer catheter very gently, the advancement is halted and the embryologist is notified. The inner catheter loaded with the embryo(s) is introduced into the outer catheter and the inner catheter is advanced toward the uterine fundus under ultrasonographic guidance. The inner catheter tip’s advancement is stalled once the distance between the distal fundal end of the cavity and the catheter tip is between 1.5 cm and 2 cm. Then the plunger of the embryo transfer syringe was pushed to complete the embryo transfer. The air bubble density can be observed at the distal fundal portion of the cavity. Right after transfer, the inner catheter is gently withdrawn while still pressing the plunger and turning the inner catheter around its axis while withdrawing. When the level of outer catheter is reached, both catheters are removed out of the cervix and returned back to the embryologist. Once the embryologist indicates the absence of any stuck embryo, embryo transfer is completed by removing the speculum and repositioning the patient to the dorsal supine position. Although studies showed no benefit of prolonged or even short rest after the embryo transfer [102–106], we keep patients lying down in mild Trendelenburg position for 10 minutes or less while reviewing post-transfer recommendations and answering all their questions. Then the patient gets up and walks to the bathroom to empty the bladder and gets dressed. We recently stopped using oral anxiolytics like diazepam 5 mg tablet before the FET. Therefore, patients can just walk out of the clinic without needing assistance. Regular daily activities are allowed after the transfer.
21.6 Conclusion
There is a growing body of evidence that in high responders and patients with PCOS [107], FET cycles have a significantly higher or comparable clinical pregnancy rates compared to fresh transfers [1, 108–110] and that FET is at least equivalent in normal responders. The choice of which FET cycle to use for a patient can sometimes be a difficult one. Patient factors such as compliance, comorbidities, and previous reproductive history must be considered along with clinic factors that relate to frequency of lab checks and what medications and protocols to follow. While sometimes taxing for a busy clinical practice, natural cycle embryo transfers are an attractive option for patients looking to minimize interventions, interested in a more “natural” approach, and looking to reduce cost on medications and avoid medication side effects. Medicated cycles, on the other hand, can be advantageous for those with irregular/unpredictable cycles and for high-volume practices that need precise timing of embryo thaw and transfer. We believe that consistency and the same careful attention paid during the ovarian stimulation phase is the key to maintaining high pregnancy rates in frozen-thaw cycles. This may be of even more importance for women with ARA and DOR as the margin of error for a successful outcome is likely to be much smaller than with good prognosis patients.

Full access? Get Clinical Tree


