Ectopic Pregnancy
Ectopic pregnancy, the implantation of a fertilized ovum outside the uterine cavity, is a worldwide public health concern and is a major contributor to reproductive failure and maternal morbidity and mortality. Providers of women’s health care need a thorough understanding of the factors that contribute to the development of ectopic pregnancy, current methods for early diagnosis, and modern treatment strategies. Early diagnosis and successful management can have a major impact upon morbidity, eliminate mortality, and may preserve future reproductive potential.
EPIDEMIOLOGY AND INCIDENCE
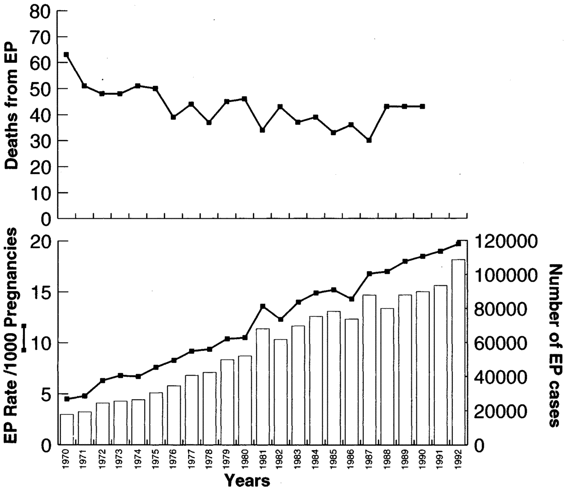
Ectopic pregnancy has increased at a staggering pace and now accounts for two percent of all pregnancies in the United States. As Figure 21-1 shows, there has been a fivefold increase in the incidence of ectopic pregnancy in the United States in the last two decades. The most recent available data estimated 108,800 ectopic pregnancies in 1992, resulting in 58,200 hospitalizations at an estimated cost of $1.1 billion (Centers for Disease Control, 1995). Because many women are now treated in an outpatient setting and are not captured in hospital admissions data, it is difficult to accurately quantify the current number and incidence of ectopic pregnancies.
FIGURE 21-1. Ectopic pregnancy in the U.S., 1970–1992.
Despite a rising incidence, the number of related deaths has progressively declined. However, ectopic pregnancy remains the leading cause of maternal death in the first trimester. Ectopic pregnancy accounted for 13 percent of all pregnancy-related deaths between 1979 and 1986, 10.8 percent of all pregnancy-related deaths between 1987 and 1990, and 9 percent of all pregnancy-related deaths in 1992. Nonwhite women with ectopic pregnancy are four times more likely to die than are white women. Recent data indicates that 30–40 women die each year in the U.S. from ectopic pregnancy, and that 70 percent of these women will have seen a physician for symptoms. Data from emergency room records indicates that 40–50 percent of women with ectopic pregnancy are misdiagnosed on their initial emergency department visit.
ETIOLOGY AND RISK
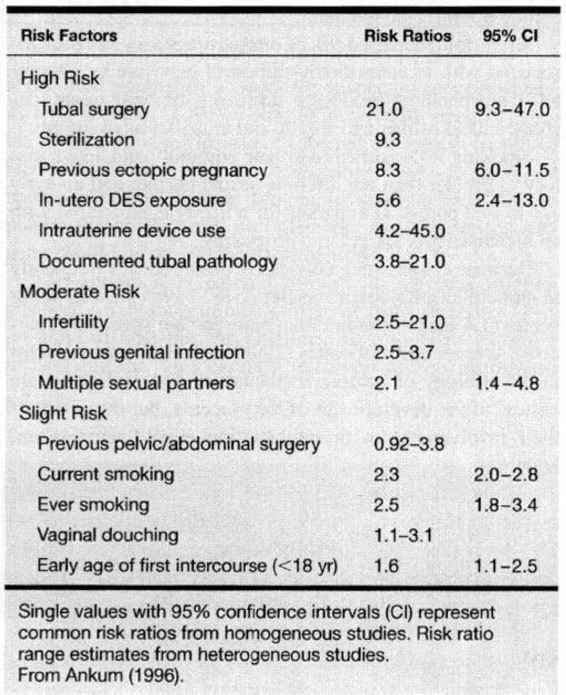
Ninety-seven percent of all ectopic pregnancies are implanted within the oviduct. Therefore, the common denominator for the majority of women who develop ectopic pregnancy is a pathologic fallopian tube. In a meta-analysis of 233 articles from 1978 through 1994 (Table 21-1), Ankum and associates (1996) reported a strong association for the development of ectopic pregnancy in women with a history of previous tubal surgery, previous ectopic pregnancy, documented tubal pathology, and in utero exposure to diethylstilbestrol. These authors reported a moderate increased risk in women with a previous history of genital tract infection, infertility, and more than one lifetime sexual partner. Only a mild increased risk was reported for women who smoked, douched, and began coitus at an early age.
TABLE 21-1. Risk Factors for Ectopic Pregnancy
SEXUALLY TRANSMITTED DISEASES
Pelvic inflammatory disease affects almost 11 percent of American women during their reproductive years and is the most common cause of tubal pathology. The causative organisms are Chlamydia trachomatis, Neisseria gonorrhoeae, and a mixture of aerobic and anaerobic organisms. Chlamydia is the most common sexually transmitted infectious disease reported to state health departments and to the Centers for Disease Control in the United States, with more than 500,000 cases reported in 1997. The reported annual rate increased almost fourfold, from 47.8 per 100,000 population in 1987 to 182.2 per 100,000 in 1995. In 1995, the reported rate for women was 290.3 per 100,000, six times higher than the reported rate for men. Unlike Neisseria gonorrhoeae and associated mixed polymicrobial infections, which can produce severe symptoms, Chlamydia trachomatis can produce minimal manifestations or be asymptomatic, but can cause extensive inflammation and scarring of the genital tract. These infections lead to deciliation of the tubal epithelium, tubal adhesions, and fimbrial agglutination.
A single episode of pelvic inflammatory disease can compromise reproductive potential. In a landmark Scandinavian cohort study from 1960 to 1984, 25 percent of all women with pelvic inflammatory disease experienced tubal infertility, and 10 percent of these women had an ectopic pregnancy with their first pregnancy. Westrom and associates (1975) reported that the incidence of tubal obstruction after one, two and three episodes of salpingitis rose from 13 to 35 to 75 percent, respectively. The probability of delivering a liveborn infant is decreased eightfold in women with recurrent and severe pelvic inflammatory disease when compared to women with mild disease.
In Sweden, where the rate of genital chlamydia has declined in the 1990s, there has been a fall in the rate of ectopic pregnancy (Bjartling and associates, 2000), giving further confirmation that pelvic inflammatory disease is linked to the future development of ectopic pregnancy.
Early diagnosis through screening of asymptomatic women can decrease the incidence of symptomatic pelvic inflammatory disease by 50 percent, and, therefore, prevent the sequelae of upper genital tract inflammation.
TUBAL STERILIZATION
At significant risk for the presence of ectopic pregnancy are women who conceive after operative tubal sterilization. These women account for 5–10 percent of all ectopics, and the literature reports 30–80 percent of conceptions after sterilization are extrauterine in location. A multicenter observational study was conducted by the Centers for Disease Control from 1978 to 1986 that followed 10,685 women who had undergone tubal sterilization for up to 10 years; Peterson and associates (1997) reported the findings of the U.S. Collaborative Review of Sterilization (CREST study). This study revealed that 32.9 percent of pregnancies occurring after prior tubal sterilization were ectopic in location. The greatest risk occurred in those women who underwent laparoscopic tubal bipolar cautery, resulting in an ectopic pregnancy risk of 17.1 per 1000 procedures, and 31.9 per 1000 procedures when bipolar cautery was used before the age of 30 years. Tuboperitoneal fistula formation is common when the fallopian tube is cauterized within 2 cm of the cornua, but fistula are less likely to occur with the use of bands and clips applied to the fallopian tube. Contrast this high rate of tubal cautery sterilization failure to the low rate of 1.5 ectopic pregnancies per 1000 procedures observed after postpartum bilateral partial salpingectomy. Ectopic pregnancy after tubal sterilization is more common in black women and in women with a history of pelvic inflammatory disease before the sterilization procedure. Yamada and Kasamatsu (2000) have presented an interesting case of bilateral tubal pregnancy in a woman following puerperal tubal ligation.
TUBAL SURGERY
Previous tubal surgery, whether for reconstruction or removal of an ectopic pregnancy, increases the risk of ectopic pregnancy 21-fold. The greatest risk is for women with tubal pathology resulting from the previous surgical attempt to correct tubal infertility, to remove tubo-ovarian adhesive disease secondary to infection and endometriosis, to remove an ectopic pregnancy, or to reanastomose a prior sterilization procedure.
PREVIOUS ECTOPIC PREGNANCY
Ten to 30 percent of pregnancies following an ectopic pregnancy are repeat ectopic pregnancies, regardless of how the initial ectopic pregnancy was treated. Damage to the contralateral fallopian tube is probably the most important contributing factor in predicting repeat ectopic pregnancy. The proportion of women with a history of a single ectopic pregnancy who subsequently become pregnant varies from 50 to 80 percent, with up to one-third being repeat ectopic pregnancies. The frequency of repeat ectopic pregnancy decreases with subsequent pregnancy order. In a study from Norway, Skjeldestad (1998) questioned women with a history of ectopic pregnancy and found that 15.9 percent of their next pregnancies were ectopic in location. However, if the next pregnancy was intrauterine only 5 percent of the third and 1.8 percent of the fourth were ectopic in location.
INTRAUTERINE DEVICES
Intrauterine devices (IUDs) prevent pregnancy by producing a sterile inflammatory response within the uterine cavity, and, to a lesser extent, within the fallopian tube, changing their cellular and hormonal fluid components. Copper ions further enhance this inflammatory response. This altered uterine and tubal environment is toxic to sperm. Therefore, the mechanism of action of an IUD is to prevent fertilization and not to act as an abortifacient.
When evaluating the threat of ectopic pregnancy in current IUD users, the risk depends upon control group comparisons. There should be a distinction between conditional risk (the risk that a given pregnancy will be an ectopic pregnancy) and absolute risk (the risk that a given woman will experience an ectopic pregnancy). The risk that an IUD user has an ectopic pregnancy is increased when compared to pregnant controls, but there is no increased risk, and probably a decreased risk, when compared to nonpregnant controls. In general, the more effective a contraceptive method is in preventing pregnancy, the lower the risk for ectopic pregnancy. The risk of ectopic pregnancy among women wearing a copper-containing IUD is more than 90 percent lower than among sexually active women using no contraception. However, because an IUD prevents intrauterine pregnancy more effectively than it prevents extrauterine implantation, a pregnancy occurring with an IUD in place is more often an ectopic pregnancy.
Progesterone-containing intrauterine devices, used less frequently for contraception, are associated with an increased risk of ectopic pregnancy. They have an overall contraceptive failure rate six times that of copper devices, with 16 percent of such pregnancies being ectopic in location.
There may be a slight increased risk for the future development of ectopic pregnancy in past IUD users, and that risk may increase with the duration of use.
INFERTILITY AND ASSISTED REPRODUCTIVE TECHNOLOGIES
Infertility poses a moderate increased risk for ectopic pregnancy which is attributed to the underlying cause of the infertility, usually tubal pathology. Controlled ovarian hyperstimulation has been implicated as a risk factor independent of the cause of infertility. The Society of Assisted Reproductive Technology (1993), through the National IVF Registry, reported that the ectopic pregnancy rate per clinical pregnancy was 5.5 percent for in vitro fertilization, 2.9 percent for gamete intrafallopian transfer, and 4.5 percent for zygote intrafallopian transfer in 1991. The overall risk of ectopic pregnancy with IVF and GIFT decreased to 2.8 percent in 1995. Estrogens increase smooth-muscle activity and muscular tone in the isthmus of the fallopian tube, which may facilitate the retention of a fertilized ovum in the ampullary portion of the tube for a few days. Progesterone decreases smooth-muscle activity, particularly tubal peristalsis. Therefore, proper fertilized ovum transport through the fallopian tube to implant within the endometrial cavity may require an optimum ratio of estrogen and progesterone.
OTHER RISK FACTORS
Kendrick (1997), Ankum (1996), and their associates reported an association between ectopic pregnancy and vaginal douching in black women, but could not distinguish douching as an independent factor from being an agent promoting a preexisting infection by forcing nonsterile fluid and organisms into the fallopian tubes. Wolner-Hanssen and associates (1990) found current douching more common among women with pelvic inflammatory disease than among noninfected women.
A five-fold increased risk of ectopic pregnancy has been associated with in utero diethylstilbestrol exposure by altering tubal morphology, producing decreased fimbrial tissue, decreased tubal caliber and length, and a smaller tubal ostium.
Smoking is presumed to impair immunity and, like a history of greater than one lifetime sexual partner and an early age at first coitus, is a marker for a lifestyle associated with an increased risk for ectopic pregnancy.
Because ectopic pregnancies are observed less frequently in gamete intrafallopian transfer (GIFT) cycles using donor sperm (1.4 percent) rather than male partner sperm (5.3 percent), Warnes and associates (1998) suggested a male factor in the etiology of ectopic implantation. Paternal genes are critical to the development of the placenta, but the extent of their involvement in preimplantation events remains unknown.
Cohen and associates (1993) performed karyotype analysis on chorionic villi from ectopic pregnancies and found 78.3 percent of 60 successful analyses abnormal. Karikoski and associates (1993) also found DNA aneuploidy by flow cytometry in one-third of paraffin-embedded ectopic pregnancy samples. Although these authors suggest that chromosomal abnormalities and abnormal embryogenesis are etiologic factors in ectopic implantation, other investigators have been unable to confirm such an association. Elias (1981), Han (1995), Job-Spica (1996), Block (1998), and their associates performed karyotype analysis on ectopic conceptuses and found no higher rate of abnormality than with failed intrauterine pregnancies.
Factors that do not seem to influence the risk of ectopic pregnancy include oral contraceptive use, previous elective pregnancy interruption, and prior cesarean delivery.
PATHOLOGY AND ANATOMY
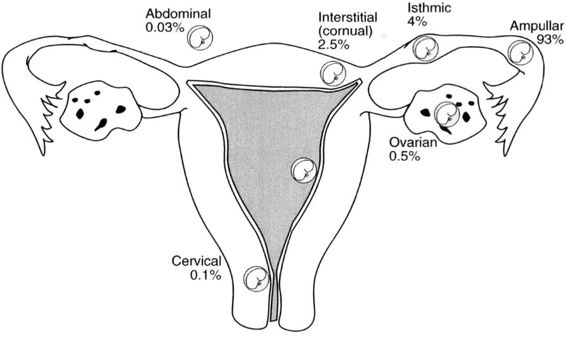
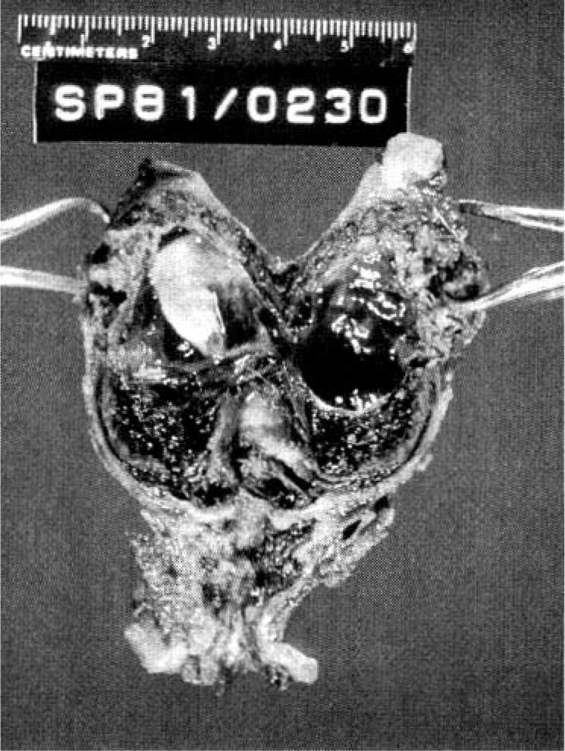
Approximately 97 percent of ectopic pregnancies implant in the fallopian tube (Fig. 21-2), and the majority of these are in the distal half of the tube; 2.5 percent are in the cornua of the uterus, and 2.5 percent are in other locations, which include the ovary, cervix, and abdomen. Most tubal ectopics are implanted in the ampullary portion of the tube (Figs. 21-3 and 21-4). This section of the tube has a large epithelial surface area, loose connective tissue, and a poorly defined muscularis. In the ampullary portion of the tube, the ectopic rapidly erodes through the tubal epithelium into the loose adventitious tissue and becomes extraluminal. The isthmic portion of the tube is narrow, with a compact, well-defined muscularis, and an ectopic implantation here grows within the lumen producing more damage to the epithelium and muscularis with earlier rupture.
FIGURE 21-2. Site and incidence of ectopic pregnancy.
FIGURE 21-3. Ruptured ampullary tubal ectopic pregnancy treated by salpingectomy.
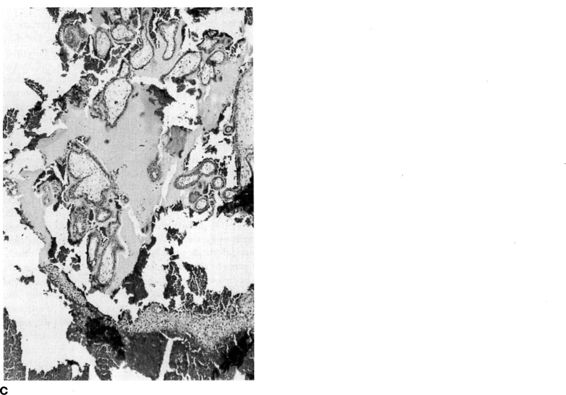
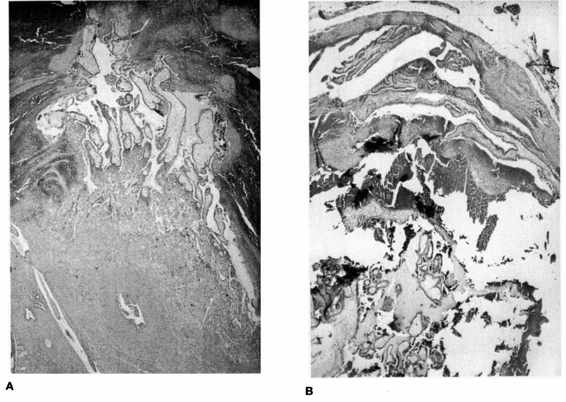
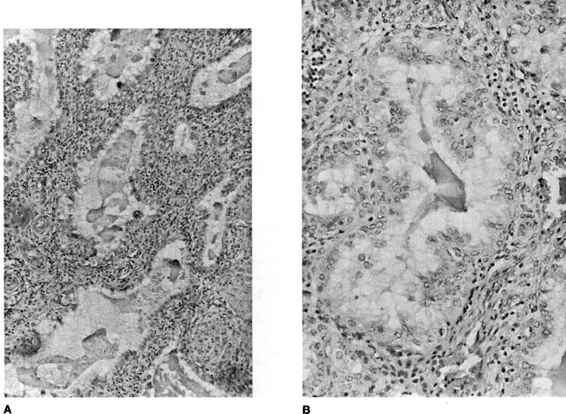
FIGURE 21-4. Photomicrograph of tubal ectopic pregnancy. A. Low-power view of tubal ectopic implantation site with chorionic villi invading tubal wall. B. and C. Higher power views of chorionic villi with tubal epithelium demonstrating lack of decidual layer characteristic of ectopic implantation. (Photos courtesy of Mary S. Richardson, MD.)
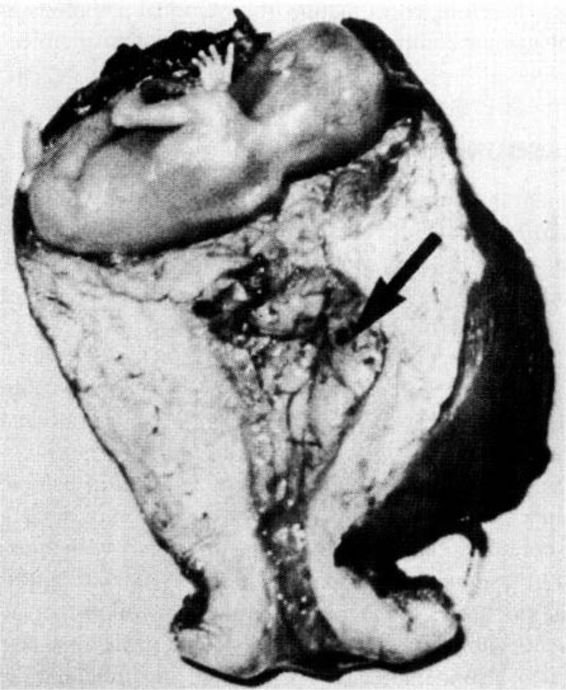
The interstitial portion of the fallopian tube is approximately 1 cm long and is surrounded by myometrium. This portion of the tube can accommodate a greater distensibility with more trophoblastic invasion and increased vascularity, with ectopic implantation potentially leading to rupture at a more advanced gestational age. Interstitial ectopic pregnancies (Fig. 21-5) are usually diagnosed late, rarely before the onset of symptoms of rupture, and are associated with increased morbidity and mortality.
FIGURE 21-5. Interstitial pregnancy.
Ovarian pregnancies are rare (less than 1 percent of ectopic pregnancies), with their etiology unclear, but have been associated with IUD use. Spiegelberg (1878) formalized the criteria for the diagnosis of ovarian pregnancy, requiring that (a) the fallopian tube on the affected side must be normal; (b) the gestational sac must occupy the normal position of the ovary; (c) the gestational sac must be connected to the uterus by the ovarian ligament; and (d) ovarian tissue must be identified histologically in the sac wall.
The cause of cervical pregnancy (Fig. 21-6), even less common than ovarian pregnancy, is unknown, but predisposing conditions include previous cervical dilatation and curettage, induced abortion, leiomyomata, Asherman syndrome, previous in utero exposure to diethylstilbestrol, presence of an intrauterine device, and in vitro fertilization. Rubin (1911) formalized the criteria for the pathologic diagnosis of cervical pregnancy. Rubin’s criteria were that: (a) cervical glands must be present at the placental attachment site; (b) the attachment of the placenta to the cervix must be below the entrance of the uterine vessels; (c) fetal parts must not be present within the uterine corpus; and (d) the placental attachment to the cervix must be intimate. Paalman and McElin (1959) added the clinical diagnostic features of cervical pregnancy, which are that: (a) the internal cervical os must be closed; (b) products of conception must be completely confined within and firmly attached to the endocervix; (c) uterine bleeding occurs in the absence of significant cramping; and (d) a soft enlarged cervix equal to or larger than the uterine fundus is palpable.
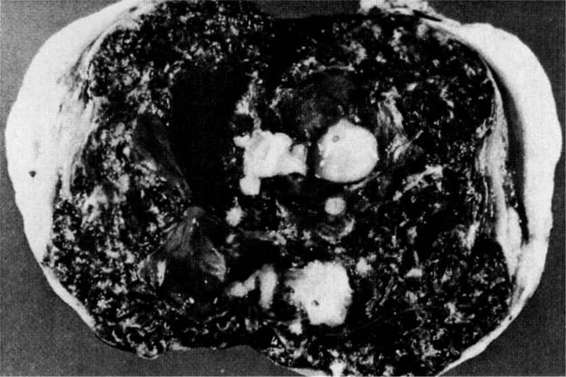
FIGURE 21-6. Cervical pregnancy.
Abdominal pregnancy, which is less than 1 percent of ectopic implantations, usually results from tubal abortion and secondary nidation. Studdiford (1942) defined the criteria for a primary abdominal pregnancy as (a) normal fallopian tubes and ovaries; (b) no evidence of a uteroperitoneal fistula; and (c) must be diagnosed early enough to eliminate the possibility of a secondary nidation.
Heterotopic pregnancy, which is the coexistence of both intrauterine and extrauterine pregnancy, is more common than originally presumed. The rate of heterotopic pregnancy was presumed to be 1 in 30,000 pregnancies, based on the frequency calculations of DeVoe and Pratt (1948). However, Bello and associates (1986) reported an actual incidence of 1 per 4000 spontaneous pregnancies, and Doyle and associates (1991) reported an incidence of 1–3 percent in pregnancies occurring after assisted reproduction.
DIAGNOSIS
THINK ECTOPIC
The early diagnosis of ectopic pregnancy is aided by a high index of suspicion, and every sexually active reproductive-age woman presenting with abdominal pain or vaginal bleeding should be screened for pregnancy.
Traditionally, the diagnosis of ectopic pregnancy has been made based on clinical signs and physical symptoms. The classic triad of an episode of amenorrhea followed by vaginal bleeding and abdominal pain is suggestive of ectopic pregnancy. Vaginal bleeding occurs because extrauterine implantations produce amounts of human chorionic gonadotropin inadequate to maintain normal corpus luteum function and its production of progesterone. Low progesterone levels lead to involution of the uterine decidua, and bleeding occurs. Abdominal pain is the result of tubal abortion or frank tubal rupture. After a woman with a positive pregnancy test demonstrates evidence of an acute abdomen and hypovolemia, the diagnosis of ruptured ectopic pregnancy seems obvious, and laparotomy may be necessary. However, the modern goal is to diagnose and treat ectopic pregnancy when conservative treatment options are possible.
Unfortunately, 40–50 percent of women with ectopic pregnancy are misdiagnosed upon initial presentation. Why is the condition so frequently missed? The elicited history of pelvic and abdominal pain and vaginal bleeding is inconsistent. Many women consider any vaginal bleeding a menstrual period, contributing to the confusion regarding amenorrhea and suspected pregnancy. The pelvic exam is insensitive in yielding an adnexal mass. In a prospective observational study from an academic urban emergency department, Dart and associates (1999) found that a palpable adnexal mass was more likely a corpus luteum cyst associated with a normal pregnancy. Significant pelvic tenderness is also insensitive in the absence of ectopic rupture, and lack of tenderness may lead to false reassurance. Sixty percent of women have no vaginal bleeding when the ectopic pregnancy is unruptured. Less than 50 percent of women with ectopic pregnancy give a history of a preexisting condition considered to be a risk factor for the development of ectopic pregnancy. The history of passed tissue can be considered the spontaneous abortion of a failed intrauterine pregnancy, when that tissue may have been a decidual cast. Low or falling serum human chorionic gonadotropin values can be misinterpreted as evidence of a failing intrauterine gestation, and a high serum human chorionic gonadotropin (HCG) level may be falsely considered a normal pregnancy. Culdocentesis, the transvaginal needle aspiration of cul-de-sac fluid, which is rarely done today, can be nondiagnostic when no fluid is retrieved and even negative, with retrieval of straw-colored peritoneal fluid, in the absence of tubal rupture. Ultrasound findings may be indeterminate, demonstrating neither an intrauterine gestation nor adnexal pathology and may not be correlated with the clinical history or with HCG levels.
The clinician cannot reliably diagnose or exclude ectopic pregnancy with history and physical exam alone, and 50 percent of all ectopic pregnancies occur in presumed low-risk patients. Therefore, confirmation of gestational pathology is made by using a combination of ultrasound and biochemical parameters.
ULTRASOUND
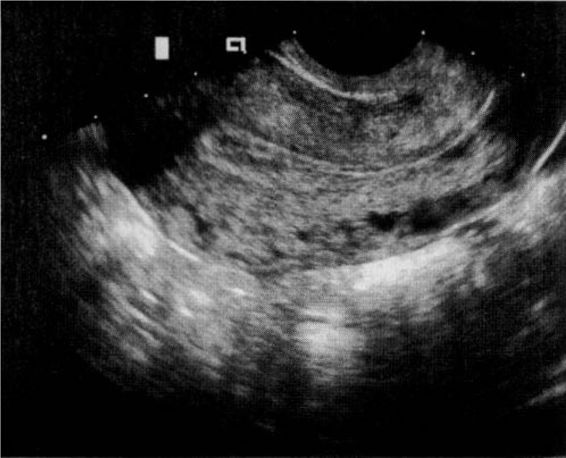
The sonographic diagnosis of ectopic pregnancy is reliably established by the direct visualization of an extrauterine gestational sac (Fig. 21-7). However, such findings rely on the skill and experience of the ultrasonographer and are evident in only a minority of cases.
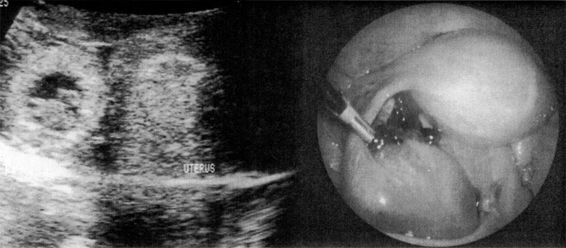
FIGURE 21-7. Transvaginal ultrasound visualization of an unruptured ampullary tubal pregnancy and appearance at laparoscopy.
With the exception of heterotopic pregnancy, which occurs in only 1 per 4000 spontaneous pregnancies, but more frequently with pregnancies resulting from assisted reproduction, the identification of an intrauterine pregnancy with ultrasound eliminates the possibility of ectopic pregnancy.
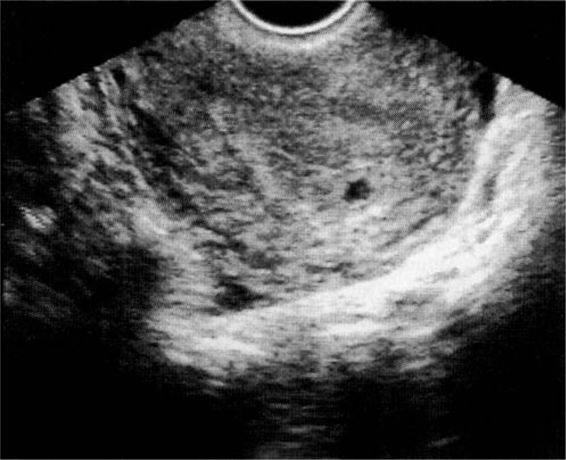
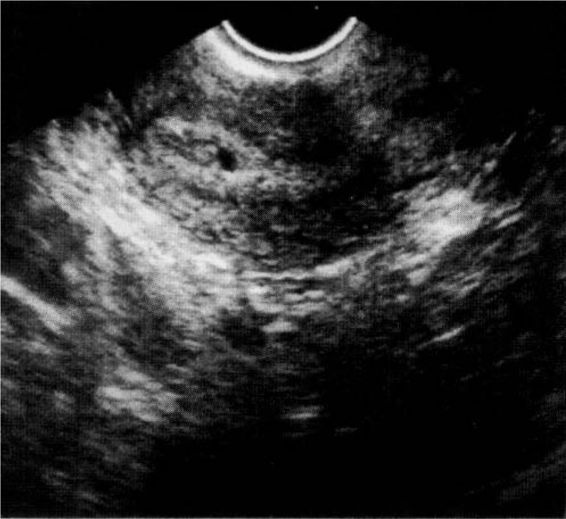
The confirmation of an intrauterine pregnancy with transvaginal ultrasound relies upon recognition initially of a gestational sac and, soon thereafter, structures within the sac consistent with a developing embryo. The gestational sac (Fig. 21-8), a sonographic term and not an anatomic term, is a thick echogenic rim surrounding a sonolucent center (a trophoblastic decidual reaction surrounding the chorionic sac) and is recognized by transvaginal ultrasound soon after the missed menses. What has been called a pseudosac (Fig. 21-9), is a collection of fluid within the endometrial cavity created by bleeding from the decidualized endometrium associated with an extrauterine implantation, and should not be mistaken for a normal early intrauterine pregnancy. The true early gestational sac is eccentrically placed within the uterus beneath the endometrial surface, the intradecidual sign, whereas the pseudosac conforms to the endometrial cavity itself.
FIGURE 21-8. Transvaginal ultrasound appearance of an early intrauterine gestational sac.
FIGURE 21-9. Transvaginal ultrasound appearance of an intrauterine pseudosac.
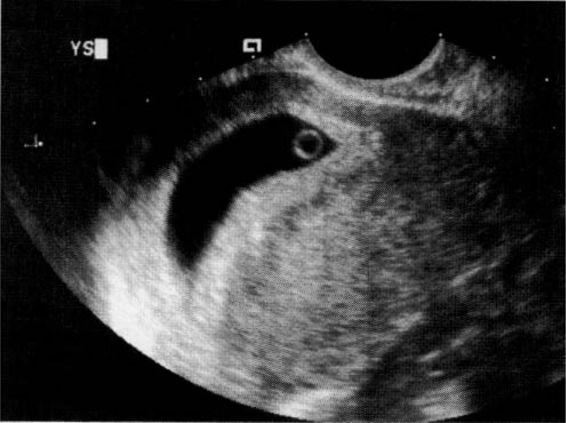
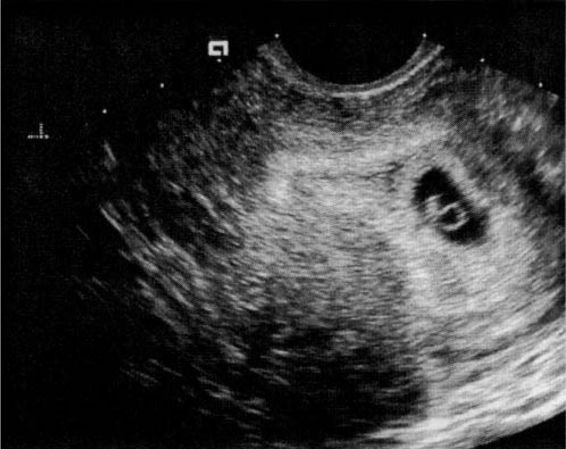
The yolk sac (Fig. 21-10), the first visible structure within the gestational sac, is a distinct circular structure with a bright echogenic rim and sonolucent center, and is recognized 3 weeks after conception (5 weeks after the last menstrual period in a 28-day cycle). The embryo (Fig. 21-11) is first recognized as a thickening along an edge of the yolk sac, and embryonic cardiac motion can be first observed 3.5–4 weeks after conception (5.5–6 weeks after the last menstrual period). A normal yolk sac is 3–5 mm in diameter, and is considered abnormal if it is greater than 7 mm in diameter. In a well-dated pregnancy with reliable ovulatory timing, an intrauterine pregnancy should be recognized with transvaginal ultrasound by 24 days after ovulation, or 38 days after the last menstrual period. With such reliable pregnancy dating, Kadar and associates (1994) suggest that the detection of a gestational sac with transvaginal ultrasound is better predicted by gestational age than by HCG levels, and that this detection is similar with singleton and multiple gestations. Failure to visualize a gestational sac 24 days after conception can be taken as presumptive evidence of ectopic pregnancy. Also, a gestational sac greater than 10 mm in diameter without a yolk sac, and greater than 16 mm without an embryonic pole, is probably abnormal.
FIGURE 21-10. Transvaginal ultrasound appearance of an early intrauterine gestational sac with a yolk sac.
FIGURE 21-11. Transvaginal ultrasound appearance of a gestational sac with a yolk sac and early embryo.
Pregnancy causes a decidual reaction within the endometrium, leading to sonographic thickening, which theoretically occurs regardless of the location of the pregnancy. Because the endometrium is so easily seen with transvaginal ultrasound, an ultrasound endometrial marker in very early gestation, before visualization of á gestational sac, similar to the characteristic histologic Arias-Stella phenomenon (Fig. 21-12), would be a useful aid in the diagnosis of ectopic pregnancy. In a small study of 117 women, Spandorfer and Barnhart (1996) reported statistically different endometrial stripe thicknesses between women with normal intrauterine, failed intrauterine, and ectopic gestations. In their report, women with normal pregnancies had endometrial stripe thicknesses of 13.42 ± 0.68 mm, whereas those with failed intrauterine and ectopic gestations measured 9.28 ± 0.88 mm and 5.95 ± 0.35 mm, respectively (p<0.01). In this report, 97 percent of women with a stripe ≤8 mm had abnormal pregnancies, with 71 percent of these abnormal pregnancies ectopic in location. Only 41 percent of women with a stripe thickness greater than 8 mm were abnormal, and only 14.7 percent were ectopic in location. No woman with an endometrial stripe thickness greater than 13 mm had an ectopic pregnancy, and no woman with a stripe thickness less than 6 mm had a normal pregnancy.
FIGURE 21-12. A. and B. Arias-Stella Phenomenon. Hypersecretory endometrium with dilated, tortuous glands with minimal intervening decidualized stroma and no chorionic villi. (Photos courtesy of Mary S. Richardson, MD.)
Lavie and associates (1996) reported that the transvaginal ultrasound recognition of a normal three-layer endometrial pattern was 62.2 percent sensitive and 100 percent specific for ectopic pregnancy. The endometrial three-layer pattern (Fig. 21-13) consists of three hyperechoic layers separated by two intervening hypoechoic layers, and is the appearance of late proliferative phase endometrium. It is believed that the outer hyperechoic layers correspond to the basal endometrium, and the middle hyperechoic layer corresponds to the apposition of the two hypoechoic layers, the edematous superficial endometrium. Wachsberg and Karimi (1998), however, reported only a 10.2 percent sensitivity and 85.2 percent specificity for ectopic pregnancy with the appearance of a thin endometrium and a normal three-layer pattern, with positive and negative predictive values of only 40 percent and 50 percent, respectively. Mehta and associates (1999) found that although there was a tendency for normal intrauterine pregnancies to have a thicker endometrial stripe, there was too much overlap in endometrial thickness to distinguish between early normal intrauterine pregnancies, failed intrauterine pregnancies, and ectopic pregnancies. In their view, these latter authors did not consider endometrial thickness helpful in confirming the diagnosis of ectopic pregnancy.
FIGURE 21-13. Transvaginal ultrasound appearance of a three-layer endometrial stripe.
HUMAN CHORIONIC GONADOTROPIN
HCG is a glycoprotein composed of two subunits, α and β. The molecular composition of the a subunit is shared by several other glycoprotein hormones, but the β subunit is unique to HCG. Antibodies against the β subunit have been produced and used as the basis for radioimmunoassays and monoclonal antibody assays.
In the absence of absolutely reliable menstrual and ovulatory dating, the concept of a discriminatory zone of HCG must be used to validate ultrasound findings. The discriminatory zone of HCG is that level of HCG at which all normal intrauterine pregnancies should be recognized. With abdominal ultrasound, that level is 6500 mlU/mL (WHO Third International Standard or International Reference Preparation as measured by radioimmunoassay), but high-resolution transvaginal ultrasound has reduced this level to 1500–1800 mlU/mL. If transvaginal ultrasound fails to recognize an intrauterine pregnancy when the HCG level has reached the discriminatory zone, the pregnancy can generally be considered extrauterine. An exception to this would be multiple gestations. Kadar and associates (1994) reported normal multiple gestations with HCG levels over 2600 mlU/mL before transvaginal ultrasound recognition. Therefore, if a multiple gestation is a possibility, such as pregnancies resulting from assisted reproduction, the HCG discriminatory zone should be raised.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree