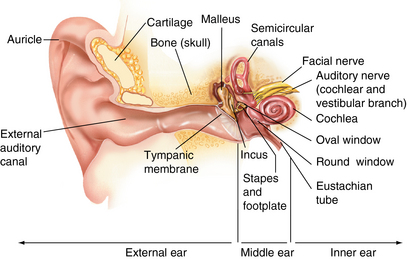
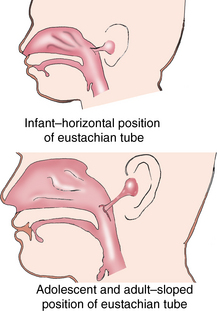
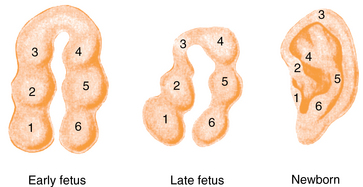
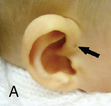
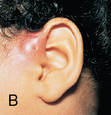
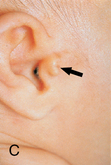
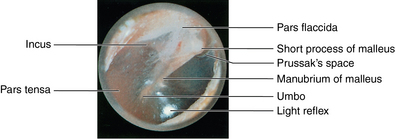
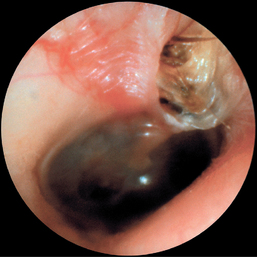
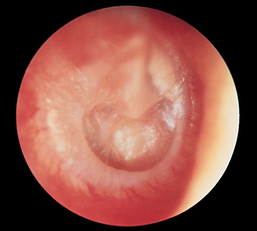
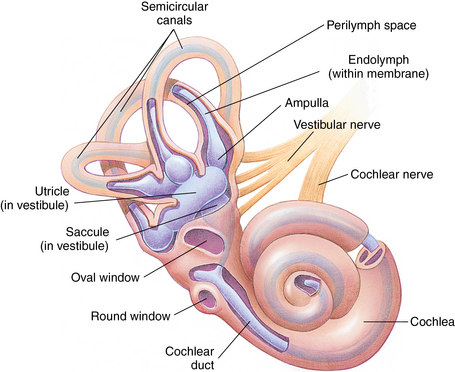
CHAPTER 12 The ear is located in the temporal bone of the skull and is composed of the inner, middle, and external ear (Figure 12-1). The structures of the ear evolve in the mesoderm, and development of the external ear begins during the sixth week of gestation when the six hillocks of His develop from the first and second branchial arches. The individual portions of the auricle, or flap of the ear, begin to fuse and assume the classic adult shape by the twelfth week of gestation, and fusion is complete by the twentieth week (Figure 12-2). The normal auricle should be no greater than 10 degrees off vertical plane or slope, and the superior portion should be in line with the outer canthus of the eye (Figure 12-3). Minor malformations of the auricle may be normal variants such as preauricular skin tags, a preauricular sinus or Darwin tubercle, a slight thickening or nodule at the upper portion of the helix (Figure 12-4). Malformations can also be the result of intrauterine position or may be related to genetic syndromes or other alterations in development occurring during the same gestational period. FIGURE 12-2 Auricular development in the fetus. (Adapted from Flint P, Haughey B, Lund V, et al: Cummings otolaryngology–head and neck surgery, ed 5, Philadelphia, 2011, Mosby.) FIGURE 12-4 A, Preauricular sinus. B, Abscessed preauricular sinus. C, Preauricular skin tags. (A, From Department of Otolaryngology, Southampton General Hospital: The preauricular sinus: a review of its aetiology, clinical presentation, and management, Int J Pediatr Otorhinolaryngol 69(11):1469-1474, 2005; B, From Zitelli BJ, Davis HW: Atlas of pediatric physical diagnosis, ed 4, St. Louis, 2002, Mosby; C, Adapted from Field et al: Pediatrics: an illustrated color text, Edinburgh, 1997, Churchill Livingstone.) Although the external ear formation coincides with the gestational formation of the internal ear structures, they develop separately. The auditory placode and the acousticofacial ganglion are present the fourth week of gestation. Over the next month, the first of the three turns in the cochlea develops. Arrest in development during this phase results in a common bony abnormality of the inner ear associated with congenital sensorineural hearing loss known as Mondini deformity. The final 2.5 turns of the cochlea occur by the twelfth week of gestation. The organ of Corti develops from the epithelium of the cochlea and is responsible for transmission of sound impulses to the eighth cranial nerve, the acoustic nerve. Improper development of the membranous labyrinth of the organ of Corti results in a Scheibe deformity, the most common congenital abnormality of the cochlear duct, resulting in sensorineural hearing loss (see later section, Hearing Loss). The semicircular canals first appear in the sixth week of gestation with differentiation of canal structures being complete by the sixteenth week. The sensory cells needed for equilibrium actually attain adult size by the twenty-third week of gestation. Alterations in development during this period due to chromosomal abnormalities or other causes can lead to hearing loss of particular tones. Simultaneous with the early development of the inner ear is the formation of the first pharyngeal pouch in the oropharynx. The proximal portion of the pharyngeal pouch develops into the eustachian tube, and the distal portion becomes the tympanic cavity and supporting structures. The eustachian tube is 17 to 18 mm long at birth and lies 10 degrees off the horizontal plane. The development of the tympanic cavity with ossicles is not complete until the eighth month of gestation, but the malleus and incus reach adult size and shape by the eighteenth week of gestation and the stapes by the twentieth week of gestation.1 The manubrium, malleus, and stapes and their supporting ligaments are formed by the Meckel and Reichert cartilage. Failure of ligaments to form properly results in a conductive hearing loss2 (see later section, Hearing Loss). A conductive hearing loss is also seen in children with osteogenesis imperfecta, which causes otosclerosis, an abnormal destruction and redeposition of the bony structures of the labyrinth. Table 12-1 presents variations in the pediatric age-group from the preterm infant to the school-age child. TABLE 12-1 Developmental Variations of the Ear The external ear is divided into sections: the outer portion is called the helix, just medial and parallel to the helix is the antihelix, and the concha is the cavity leading to the opening of the external canal (Figure 12-5). A firm protuberance on the anterior portion of the ear just at the entrance to the auditory canal is the tragus, and across from the tragus on the border of the antihelix is the antitragus. Beneath the tragus is the soft fold of skin that forms the ear lobe. Although the shape of the auricle varies slightly from person to person, the ears should be comparable bilaterally in size, shape, and position and not significantly varied bilaterally. FIGURE 12-5 Anatomy of the external ear of small child labeled with anatomy. (From Zitelli BJ, Davis H: Atlas of pediatric physical diagnosis, ed 5, St. Louis, 2008, Mosby.) The tympanic membrane is a thin layer of oval-shaped skin attached to the wall of the external canal and is approximately 9 to 10 mm in diameter (Figure 12-6). It is surrounded by a fibrous band called the annulus. The medial surface of the tympanic membrane is attached to the manubrium and lateral process of the malleus. The tympanic membrane has a resonance frequency of 800 to 1600 Hz, approximating the normal speech frequency of 500 to 2000 Hz found in humans. The tympanic membrane is divided into sections: (1) the pars flaccida is superior to the lateral process of the malleus, (2) the pars tensa comprises the majority of the tympanic membrane inferior to the lateral process of the malleus, and (3) Prussak’s space, which lies medial to the pars flaccida in the anterior superior quadrant of the tympanic membrane (see Figure 12-6). Prussak’s space is the most common location of retraction pockets and congenital or acquired cholesteatoma (Figure 12-7), an asymptomatic white mass in the middle ear that is thought to arise from the continued growth of the epidermoid layer over the tympanic membrane.1 Tympanosclerosis, thickening and scarring of the tympanic membrane, is commonly seen after chronic infections of the middle ear (Figure 12-8). FIGURE 12-6 Normal left tympanic membrane. (From Seidel HM, Ball JW, Dains JE, et al: Mosby’s guide to physical examination, ed 7, St. Louis, 2011, Mosby.) FIGURE 12-7 Cholesteatoma. (Adapted from Flint P, Haughey B, Lund V, et al: Cummings otolaryngology–head and neck surgery, ed 5, Philadelphia, 2011, Mosby.) FIGURE 12-8 Scarring on the tympanic membrane. (From Zitelli BJ, Davis H: Atlas of pediatric physical diagnosis, ed 5, St. Louis, 2008, Mosby.) The three ossicles of the inner ear, the malleus, incus, and stapes, the smallest bones in the body, transmit the movement of the tympanic membrane to the oval window and subsequently to the vestibular and cochlear branches of the eighth cranial nerve, the acoustic nerve (Figure 12-9). The head of the malleus articulates with the body of the incus at the incudomalleolar joint. The long crus or leglike structure of the incus, articulates with the head of the stapes at the incudostapedial joint. These joint areas are the most vascular regions of the ossicles, and therefore are the most susceptible to trauma or infection. The footplate of the stapes sits upon the oval window of the inner ear at the fibrous stapediovestibular joint. Because of the mechanical function of the three ossicles and the transmission of sound waves from the larger surface area of the tympanic membrane to the smaller surface area of the oval window, there is a net increase of 22 times the sound energy radiating from the tympanic membrane to the oval window. FIGURE 12-9 Anatomy of the inner ear. (From Thibodeau GA, Patton KT: Anatomy and physiology, ed 6, St. Louis, 2007, Mosby.) The eustachian tube opens into the oropharynx just behind the nasal cavity and is the drainage and ventilatory structure for the middle ear. The eustachian tube connects the middle ear with the back of the throat. The middle ear is an air-filled space and when patent has the same air pressure as the outside air pressure. Normally, swallowing opens the eustachian tube and restores and equalizes the pressure between the middle ear and the outside air pressure. Changes in altitude or ambient pressure, such as occurs on an airplane, can change the middle ear pressure, resulting in pain or discomfort. Repeated swallowing or in infancy sucking on the bottle or breast can often equalize the eustachian tube pressure and reduce the associated pain. In the adult, the eustachian tube averages 35 mm in length. In infancy, it is half that length (around 18 mm), which allows bacteria and viruses to more easily migrate from the oropharynx to the middle ear (Figure 12-10). The musculature of the eustachian tube, which controls function, is innervated by the motor division of the fifth cranial nerve, or trigeminal nerve. In infants and children with cleft palate and other central craniofacial abnormalities such as Down syndrome, structural or functional abnormalities of the eustachian tube interfere with normal ventilation and clearance of the middle ear, increasing the incidence of otitis media in these children. Equalization of pressure in the middle ear is critical for normal sound wave vibration of the tympanic membrane. The inner ear is the sensory end organ and is directly responsible for hearing and balance. The inner ear contains the vestibule, semicircular canals, and cochlea and is bathed in fluid that facilitates the transmission of sound waves to the auditory nerve and sensations of balance in the semicircular canals (see Figure 12-9). The sound waves pass over approximately 30,000 innervated hair cells in the cochlea, which are the primary receptors, transducers, and conveyers of sound energy to the brain. In recent years, the understanding of genetics and the auditory system have increased significantly. Autosomal recessive genetic sensorineural hearing loss (SNHL) accounts for about 80% of all SNHL in children, and autosomal dominant genetic SNHL accounts for 10%.1 Some children with hearing loss have identifiable syndromes, such as Usher syndrome, Pendred syndrome, or Jervell and Lange-Nielsen syndrome. Infants with malformed external ear structures require close monitoring. Infants identified with nonsyndromic hearing loss may be missed and are harder to diagnose. Many genetically determined causes of hearing loss do not present at birth and may not be identified through newborn screening. Radiographic imaging such as a CT scan or MRI can identify up to 40% of middle and inner ear abnormalities, but imaging is used cautiously because of the concern of long-term risks of radiation exposure for infants and young children. Genetic testing is now often the first investigative tool to identify the cause of hearing loss.3 Acquired causes of hearing loss are numerous and risk factors must be identified (Box 12-1). Prenatal risk factors include gestational diabetes in the mother, congenital infections (cytomegalovirus, rubella, toxoplasmosis, herpes, syphilis, varicella), exposure to teratogens (alcohol, methyl mercury, thalidomide, cocaine), and ototoxic medications (aminoglycosides, loop diuretics, quinine). Prematurity, birth hypoxia, hyperbilirubinemia, sepsis, and administration of ototoxic medication are risk factors for acquired hearing loss in the perinatal period. Head trauma, infections (mumps, measles, varicella, meningitis, Lyme disease), recurrent otitis media, and excessive noise exposure are risk factors for hearing loss any time they occur.3 The research literature is inconclusive on the ethnic variations in the prevalence of hearing loss or frequency of otitis media. There is research indicating an increased incidence of otitis media in Native American/Alaska Native children.4 Other risk factors for otitis media across ethnic groups include children living in low-income households and maternal smoking.4,5 There is a predisposition or genetic inheritance pattern for both otitis media and sensorineural hearing loss; therefore, family history should be carefully evaluated when gathering a comprehensive health history, and children at risk should have more frequent screening for both otitis media and hearing deficits. The Information Gathering table presents the important information to be gathered for each age-group and developmental stage. Table 12-2 presents the important information for a symptom-focused history for children or adolescents presenting with ear symptoms. TABLE 12-2 Symptom-Focused History for Ear Assessment
Ears
Embryological development
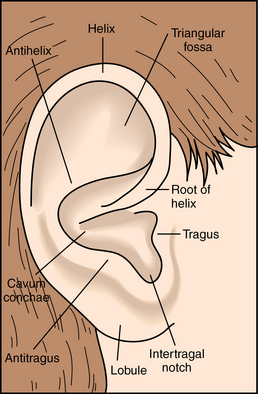
External ear
Inner ear
Middle ear
Developmental variations
Age-Group
Physiological Variations
Preterm
Vulnerable to hearing loss, particularly before 33 weeks, from noise exposure, hypoxia, ototoxic drugs, hyperbilirubinemia, persistent pulmonary hypertension
Newborn
At birth, tympanic membrane is almost adult size but lies in a more horizontal plane compared to the adult ear, which alters visual assessment
Intrauterine positioning may result in disfiguring of the pinna, which will usually resolve after birth with proper positioning because of the elastic quality of the ear cartilage
Whitish material including vernix caseosa may be present in external auditory canal
Canal narrow and curved making assessment of the middle ear difficult
Determining patency of canal is critical
Infancy
Fluid easily trapped in the middle ear due to eustachian tube dysfunction, particularly common in infants with Down syndrome, preterm infants, and any infant with craniofacial abnormalities
Early childhood
External auditory canal ossifies by 2 years of age, straightening the canal and improving visualization of tympanic membrane
The pinna is approximately 80% of the adult size in the 4- to 5-year-old
Middle childhood
In a 9-year-old, the pinna and external auditory canal have attained adult size
The canal measures 2.5 cm and has become somewhat S-shaped
Anatomy and physiology
External ear (pinna)
Middle ear
Inner ear
Physiological variations
Family, cultural, racial, and ethnic considerations
System-specific history
Symptom
Questions to Ask
Ear pain
Onset, duration, and intensity of pain?
Associated symptoms (e.g., fever, rhinorrhea, cough, ear drainage, hearing loss, vertigo, ringing in ears, swelling or redness around ear, mouth sores, dental pain, sore throat, difficulty sucking or swallowing, vomiting, neck swelling, tenderness)?
Concurrent illness (e.g., upper respiratory infection, mouth infection, skin infection)?
Home management of pain (e.g., medications/home remedies): type, how much, how often, how effective?
Changes in activities of daily living (e.g., loss of sleep, change in appetite, ability to attend daycare, school, or work)? Changes in activity level, talking, or movement of temporomandibular joint? Change in interaction with others (e.g., playful, withdrawn, irritable)?
What makes the pain feel better, worse?
Others at home, daycare, school, or work with similar symptoms?
What do you think might be the cause of the pain?
In infancy: is infant pulling at ear, showing increased irritability, feeding poorly, or waking more frequently at night?
Ear drainage
Onset, duration, and intensity of discharge?
Associated symptoms (e.g., fever, rhinorrhea, cough, ear pain, hearing loss, vertigo, ringing in ears, swelling or redness around ear, vomiting)?
Concurrent illness (e.g., upper respiratory infection, mouth infection, skin infection)?
Changes in activities of daily living (e.g., loss of sleep, change in appetite, ability to attend daycare, school, or work)? Changes in activity level, interaction with others (e.g., playful, withdrawn)?
Home management of drainage (e.g., medications/home remedies): type, how much, how often, how effective?
Injury caused by pressure or trauma (e.g., laceration or barotrauma)?
Others at home, daycare, school, or work with similar symptoms?
How do you care for/clean your child’s ears?
What do you think might be the cause of the ear drainage?
Hearing difficulty relevant in school-age child and adolescent
Gradual or sudden onset? Progressive?
Bilateral or unilateral?
Associated with other symptoms (e.g., ear pain, sense of fullness, drainage, systemic symptoms of illness)?
Concurrent illness (e.g., otitis media, otitis media with effusion, respiratory allergies)?
Trauma or exposure to loud noises?
Changes in activities of daily living (e.g., difficulty hearing in school, at home, watching television, talking on phone)?
Home management of hearing difficulty (e.g., sitting closer to television or in front of classroom, increasing visual cues for communicating)?
What conditions make hearing better or worse?
What do you think might be the cause of the hearing difficulty?
Dizziness or vertigo relevant in school-age child and adolescent
Gradual or sudden onset?
Associated with other symptoms (e.g., nausea, vomiting, tinnitus, ear pain, ear drainage, hearing loss, systemic symptoms of illness)?
Concurrent illness (e.g., viral illness, gastroenteritis, respiratory allergies/illness)?
Use of medications or recreational drugs?
Changes in activities of daily living (e.g., ability to attend school and work)?
Home management of dizziness? Others in home with similar symptoms?
What makes dizziness better or worse?
What do you think might be the cause of the dizziness?![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Ears
Only gold members can continue reading. Log In or Register to continue