Early Pregnancy Failure and Ectopic Pregnancy
Charlie C. Kilpatrick
George Verghese
Early pregnancy failure (EPF), also referred to as miscarriage or abortion, is the most common complication of early pregnancy.1 Almost one in four women will experience EPF in her lifetime. Costs associated with the management of EPF within the United States in 2002 were estimated at more than 260 million dollars.2 With the improvement in ultrasound technology, the nomenclature and diagnosis of EPF has greatly improved.
Ectopic pregnancy (EctP) diagnosis and treatment has also improved over the last several decades. This is likely due to the improvement in the sensitivity of serum beta human chorionic gonadotropin (beta-hCG) assays, contribution of high-resolution ultrasound, and the rise in the use of laparoscopy and medical methods for treatment.3 Improved technology has likely contributed to the complacency fostered with the diagnosis and treatment of EctP, but the EctP still remains a major source of morbidity and mortality for reproductive age women and is the third most common cause of pregnancy-related death.4
The ultrasound has been instrumental in more accurately distinguishing between EPF and EctP. The goal is to shape our understanding of these two common early pregnancy complications and provide a framework for how to recognize them.
TERMINOLOGY
The terminology of EPF, depending on where you are in the world, is likely to be different.5 Many terms are used in the literature to describe the failure of a pregnancy in early gestation, which can lead to confusion in patient counseling.
The term abortion likely originates from 15th century Latin “aboriri” meaning “to disappear, perish or pass away, be lost, or miscarry.”6 Originally intended to reflect both spontaneous and intentional acts, there arose a distinction between intentional and spontaneous abortion likely in the late 19th century. The term miscarriage came to be used in reference to a spontaneous event, whereas leaving the original term, abortion, associated with an intentional act. Currently, the American College of Obstetrics and Gynecology favors the World Health Organization definition of abortion, “expulsion or extraction of an embryo or fetus weighing 500 g or less from its mother.”7
The term early pregnancy failure refers to either an embryonic/fetal demise or anembryonic pregnancy.8 The broader lay term miscarriage encompasses EPF, inevitable and incomplete abortion, and any fetal loss until viability is reached.
An embryo encompasses a human offspring in the early stages following conception and implantation and up to the end of the eighth week of life.9 Studies that refer to a loss prior to 10 weeks’ gestation should refer to the loss as an embryonic loss rather than a fetal loss. A fetus is a developing human from usually 2 months after conception to birth.9 A loss after the 10th week of gestation should reference as fetal loss.
With the advent of ultrasound to make the diagnosis of pregnancy and EPF, older terminology based on physical exam findings is sometimes not helpful. The term missed abortion, where the uterus has somehow “missed” recognizing the abortion is of little help clinically. This term originated when physical exam findings did not correlate with menstrual recall, and the size of the pregnancy on physical exam was less than that expected by gestational age. Incomplete and inevitable abortions also have their origins in physical exam findings. Incomplete abortion refers to incomplete passage of embryonic/fetal tissue, whereas inevitable abortion describes a process in place that cannot be stopped. These terms have more to do with a physiologic process rather than an underlying cause.8
Early pregnancy loss (EPL) is another term sometimes used to describe the early failure of a pregnancy. EPL more specifically refers to pregnancy losses that occur before clinical recognition, so-called hidden events.10 These are conceptions that occur and fail before implantation. The patient likely would not have known she had become pregnant and passed the pregnancy at the time of her normal menses. With the development of more sensitive assays, these events have been elucidated.
INCIDENCE
EPF is the most common complication of early pregnancy. In those women with clinically recognized pregnancies, 8 to 22% will experience EPL.11 When all conceptions are taken into account, including EPL, the rate of
EPF is greater than 30%,12 a rather inefficient process. This information is useful when treating and counseling patients because it may help to allay the anxiety that is experienced by a patient undergoing miscarriage. The percentage of patients undergoing miscarriage decreases as gestational age increases,13 with about 80% occurring within the first 12 weeks.14 Miscarriage after 15 weeks in genetically normal fetuses is unusual.13 This trend in decreasing incidence with increasing gestational age is important as we discuss the risk factors and etiology for EPF.
EPF is greater than 30%,12 a rather inefficient process. This information is useful when treating and counseling patients because it may help to allay the anxiety that is experienced by a patient undergoing miscarriage. The percentage of patients undergoing miscarriage decreases as gestational age increases,13 with about 80% occurring within the first 12 weeks.14 Miscarriage after 15 weeks in genetically normal fetuses is unusual.13 This trend in decreasing incidence with increasing gestational age is important as we discuss the risk factors and etiology for EPF.
RISK FACTORS
There have been numerous studies directed at uncovering the most common risk factors for EPF. To date, the most compelling reasons are advancing maternal age and a history of pregnancy loss.15,16
In a case control study, which took place in the United Kingdom, the rate of miscarriage was 75% higher in women older than the age of 35 years, increasing to a five-fold risk of fetal loss after the age of 40 years.16 A study of the Danish Medical Birth Registry, including more than a million women that were admitted to the hospital, discovered the same trend with almost half of the women at age 40 years and older undergoing early miscarriage.15 This association between maternal age and miscarriage is depicted in Figure 12.1. Logically, this increase in maternal age-associated miscarriage can be explained by a woman’s older genetic material, but even when chromosomal abnormalities are taken into account, maternal age is an independent risk factor for miscarriage.17
Previous EPL is also a strong predictor of future miscarriage, with the odds increasing with each subsequent EPL,16 reaching a greater than three-fold increase with three prior losses. In this study, a history of a previous live birth decreased miscarriage rates by as much as 40% in subsequent pregnancies. These investigators also noted a doubling of the miscarriage rate in those women that had taken longer than 12 months to conceive compared to those who had conceived within 3 months and a higher rate of miscarriage in those women that were not married or in a stable relationship.
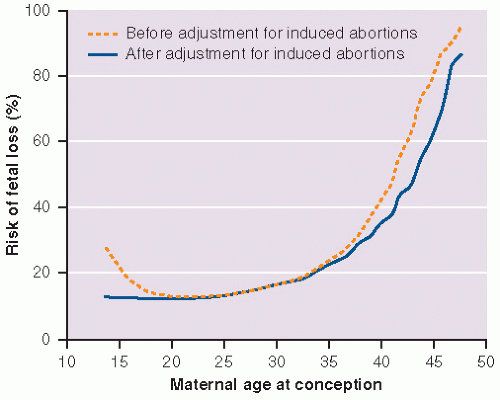 FIGURE 12.1 Risk of fetal loss from spontaneous abortion, ectopic pregnancy, and stillbirth according to maternal age at conception. |
Smoking has been linked to EPL. In a large case control study, Ness et al.18 showed an increase in the rate of miscarriage among women with positive urine cotinine (cigarette breakdown product) and positive self-report history of smoking as compared to controls. George et al.19 found a similar association among smoking and miscarriage, even reporting a link between environmental tobacco smoke (ETS) and miscarriage. In this study, they also analyzed the chromosomes of the products submitted. The association between ETS and miscarriage did not exist among miscarriage products that had a normal chromosomal analysis. Maconochie et al.16 did not find an association between smoking and miscarriage. Other risk factors that have been associated with an increased risk of miscarriage include alcohol intake, cocaine use, and maternal weight.18,20 A recent meta-analysis of overweight and obese women compared to controls noted an almost two-fold increase in the risk of miscarriage among the former.21
Endocrine causes such as diabetes and thyroid abnormalities have been linked with an increased risk of miscarriage.22,23 Infectious etiologies have not been rigorously studied to determine if they play a role in miscarriage.
Autoimmune factors have received much attention lately in regard to miscarriage. Acquired thrombophilias such as the lupus anticoagulant and anticardiolipin antibodies have been linked with second- and thirdtrimester losses.23 It remains to be seen whether this holds true within the first trimester and whether testing and treatment in those with recurrent miscarriage benefit from addressing these factors. Inherited thrombophilias including protein C, S, and G20210A have also received attention as a cause of miscarriage, but the data surrounding this is confusing, conflicting, and costly if put into practice. Currently, there is a need for additional prospective data to make recommendations in regard to screening and treatment of these disorders.
Lastly, anatomic factors such as uterine fibroids and uterine anomalies may play a role in miscarriage, but more prospective data on these relationships is needed.24
ETIOLOGY
The great majority of EPFs are due to chromosomal abnormalities or congenital anomalies not compatible with life. Congenital anomalies are usually due to an underlying genetic cause with some secondary to environmental factors (teratogens).23 Other causes of EPF are those associated with the patient such as an underlying anatomic abnormality (intrauterine septum or submucosal fibroid) or a poorly controlled endocrinopathy (diabetes, thyroid disease).23,24 Often, the explanation for miscarriage in a patient that has undergone recurrent losses cannot be determined.
Cytogenetic abnormalities are found in 50% of EPF specimens. The earlier the gestational age at which miscarriage occurs, the higher the incidence of chromosomal abnormality discovered. Abnormal karyotype may be present in as much as 90% of anembryonic pregnancies, decreasing to 50% at 8 to 11 weeks’ gestation and 30% by 16 to 19 weeks’ gestation.25 By far, the most common cytogenetic cause is autosomal trisomy (60%), followed by monosomy X (20%), and triploidy (20%).26 As pregnancy advances, the chromosomal abnormalities discovered are similar to the abnormalities seen in live born infants: trisomy 21, 18, and 13.27
CLINICAL FEATURES AND DIAGNOSIS
Women that are possibly undergoing miscarriage present with irregular bleeding and sometimes uterine cramping. These women may or may not know that they are pregnant. A urine pregnancy test is necessary for all women of childbearing age that present with unexplained vaginal bleeding. In those that are pregnant, a quantitative serum beta-hCG test and/or ultrasound can aid in determining the cause. An algorithm for approaching these patients is detailed in Figure 12.2.
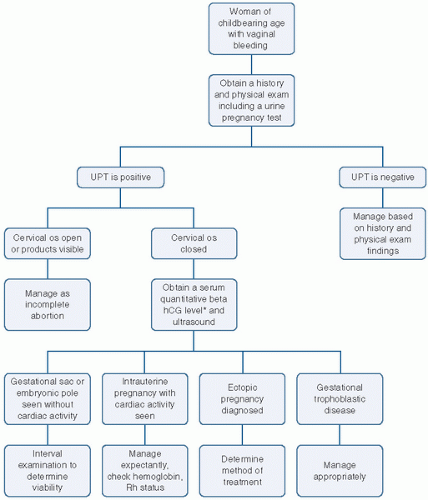 FIGURE 12.2 Algorithm for vaginal bleeding. Serum beta-hCG level should be above your institution’s threshold for ultrasonographic visualization. UPT, urine pregnancy test. |
If the woman is not pregnant, management should be based on the history and physical exam findings. If she is pregnant and appears to be in the process of aborting with products passed or at the cervical os, management should be undertaken to complete the process. An incomplete abortion may be confirmed on physical exam with discovering products of conception or even the patient bringing tissue or relating a story of passing tissue at home, and the presence of other physical findings such as an open cervical os, continued vaginal
bleeding, and cramping. The cramping associated with the miscarriage process should cease once the products are no longer in the uterus. Inevitable abortion refers to bleeding of intrauterine origin and progressive cervical dilation without passage of tissue.
bleeding, and cramping. The cramping associated with the miscarriage process should cease once the products are no longer in the uterus. Inevitable abortion refers to bleeding of intrauterine origin and progressive cervical dilation without passage of tissue.
If she is pregnant, with vaginal bleeding, a closed os, ultrasound evidence of intrauterine contents, and without history or evidence of passing tissue, the scenario is referred to as a threatened abortion. The serum beta-hCG level and ultrasound are crucial to determine whether the pregnancy is viable, failed, ectopic, or gestational trophoblastic disease.
The gestational sac is the first intrauterine structure seen on ultrasound in pregnancy. This can be visualized around 4 weeks and is usually 2 to 3 mm in size. Visualization of the gestational sac allows one to determine if a pregnancy is within the uterine cavity, eliminating the possibility of EctP except in the rare case of a heterotopic pregnancy where there exists pregnancies in two implantation sites. A heterotopic pregnancy is encountered rarely28 unless assisted reproductive techniques are employed where the incidence is higher.29
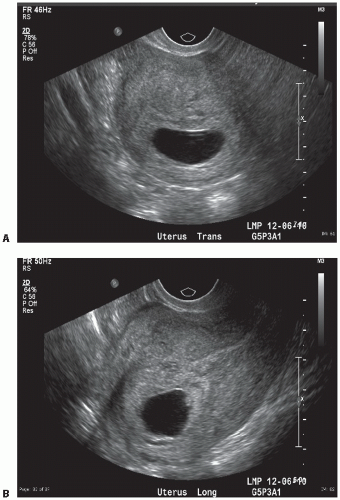
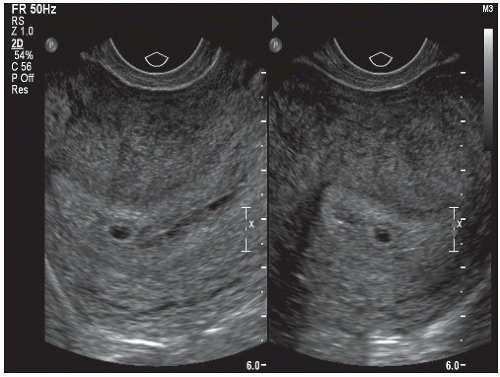
The gestational sac is visualized as an anechoic space (clear) surrounded by a hypoechoic rim (bright echoes). The sac is eccentric in its intrauterine location and spherical in shape (Fig. 12.3), which should be distinguished from the centrally located “pseudogestational sac” (Fig. 12.4).
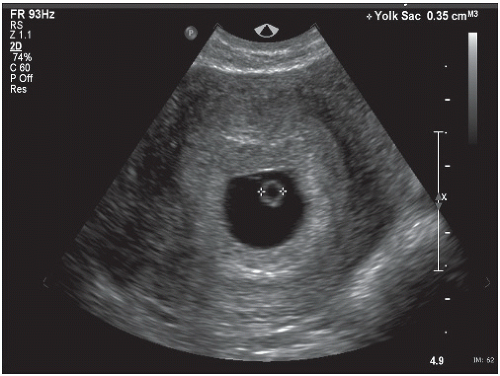
The first structure seen within the gestational sac is the yolk sac. The yolk sac can be visualized by the fifth week of gestation. The yolk sac provides nourishment to the developing embryo and is a round sonolucent structure with a bright rim (Fig. 12.5). Thickening of the margin of the yolk sac is referred to as the fetal or embryonic pole. The fetal pole is the embryo in its somite stage. A better term would be embryonic pole, but fetal pole is often used. Between 5 and 6 weeks’ gestation, fetal cardiac activity should be present within the embryonic pole.
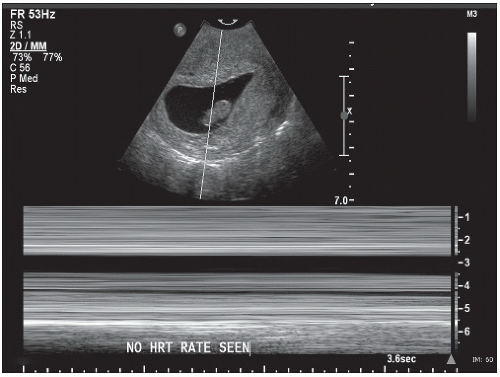
Recent ultrasound studies classify fetal demise (again the nomenclature is confusing and embryonic demise
would be more correct) once the fetal pole measures 5 mm without the presence of cardiac activity.30 In this scenario (Fig. 12.6), the embryonic disk develops, but no further discernible development is noted.8 Fetal or embryonic demise can replace the oft-used term missed abortion, which continues to linger although adds little useful clinical information.31
would be more correct) once the fetal pole measures 5 mm without the presence of cardiac activity.30 In this scenario (Fig. 12.6), the embryonic disk develops, but no further discernible development is noted.8 Fetal or embryonic demise can replace the oft-used term missed abortion, which continues to linger although adds little useful clinical information.31
Anembryonic pregnancy is an empty gestational sac measuring more than 15 or 20 mm in diameter without a fetal pole or the lack of interval growth in a smaller gestational sac on repeat sonogram after 7 days.32 In this situation, the embryonic disk does not develop or has resorbed after the trophoblast has invaded the decidual lining of the uterus8 (Fig. 12.7). Anembryonic pregnancy can replace blighted ovum, which was used to describe an empty gestational sac with no embryo.
A combination of physical exam skills, laboratory data, and ultrasound can be used to determine whether a pregnancy is a miscarriage, viable intrauterine pregnancy, or ectopic. Urine pregnancy tests are qualitative with a minimum detection level of 20 to 25 mIU/mL. Serum tests can be either, but when trying to determine viability of a pregnancy, the quantitative test is most helpful, and the minimum detection level is 1 to 5 mIU/mL. Hospital laboratories use different references and measures, making comparisons between two different sites problematic. For this reason, it is not recommended to compare quantitative beta-hCG levels between two different laboratories.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree