Drugs and Lung Development
Alan H. Jobe
John P. Newnham
Lung development is a target for drug therapy only within the context of prematurity. Corticosteroids are standard of care for women at high risk of preterm delivery to decrease the risk of respiratory distress syndrome (RDS) and increase infant survival. Corticosteroids have pleiotropic effects, which can be both beneficial and potentially harmful for the developing lung and other fetal organ systems. Although lung development and maturation are modulated by multiple hormones, growth factors, and disease states, no drug class other than corticosteroids has proven effective as a maturational agent in clinical practice. This chapter reviews the clinical literature on the use of antenatal corticosteroids to induce early lung maturation and emphasizes the areas of uncertainty. Other agents that have not proven to be effective are briefly discussed.
The Development of Antenatal Corticosteroid Therapy
In 1969, Liggins observed that fetal sheep infused with cortisol had lungs that were better aerated than control lungs after preterm delivery (1). Liggins and Howie (2) then reported in 1972 the first randomized control trial of antenatal corticosteroids, which demonstrated decreased RDS and decreased death in preterms, with no increase in complications. The trial was controversial because the obstetric community was concerned about adverse effects of “steroids” on human development after the experience with diethylstilbestrol. Over the next 18 years multiple trials were reported, which generally supported the benefits of antenatal corticosteroid treatments. In 1990, Crowley and colleagues published a meta-analysis of the trials demonstrating compelling benefit with minimal risk (3). However, in that era in the United States and elsewhere, fewer than 20% of women at risk of preterm delivery were treated with antenatal corticosteroids. In 1994, a National Institutes of Health (US) Consensus Conference strongly endorsed the use of antenatal corticosteroids (Table 19.1) (4), and utilization rates increased to current treatment rates of 80% to 90% of women at risk. However, many clinicians began using repetitive courses of antenatal corticosteroids, which resulted in a second Consensus Conference in 2000, which recommended against repetitive courses of corticosteroids until further studies were available (5). Some of those trials of repetitive dosing with maternal corticosteroids are now available and will be reviewed (6,7). Although antenatal corticosteroid treatment is considered the standard of care by obstetric societies worldwide and the NIH, the indication is not approved by the US Food and Drug Administration because no request for approval has been submitted by pharmaceutical companies.
Single-Course Treatments with Antenatal Corticosteroids
The clinical indicators for a single course of antenatal corticosteroids were developed by the NIH Consensus Conference in 1994, based primarily on the Crowley meta-analysis (3). The most recent update of that meta-analysis in 2006 by Roberts and Dalziel further strengthens the recommendations for treatment (8). This is now the definitive analysis as further placebo-controlled trials are not ethical. The analysis includes 21 randomized controlled trials of 3,885 women and 4,269 infants. No risks for the mother were identified, even for women with preeclampsia or diabetes, although attention to glycemic control is required after corticosteroids are administered to women with diabetes. The benefits for the newborn are substantial with large decreases in death, RDS, intraventricular hemorrhage, and necrotizing enterocolitis (Fig. 19.1). The risk of developing bronchopulmonary dysplasia is not decreased.
The primary indication for antenatal corticosteroid treatment in most studies was the prevention of RDS. The effect of antenatal corticosteroids on RDS is robust with comparable decreases in RDS independent of gestational age more than 28 weeks of gestation. However, because the risk of RDS decreases as gestational age increases, the number needed to treat to prevent one case of RDS was 4 for deliveries prior to 31 weeks, 15 at 31 to 34 weeks, and 145 at greater than 34 weeks (9). The treatment to delivery interval for the maximal decrease in RDS was 1 to 7 days after initiating treatment, and with loss of benefit after 7 days (Fig. 19.1). The RDS benefit was demonstrated in trials conducted in the 70s, 80s, and 90s, demonstrating persistent benefits across time.
Table 19.1 Recommendations of the 1994 and 2000 Consensus Conferences for the Use of Antenatal Corticosteroids | ||
|---|---|---|
|
The clinical literature supports other benefits, which were also demonstrated in experimental animal models (10). Preterm fetal exposure to corticosteroids increased blood pressure and myocardial performance after preterm delivery. The blood pressure was higher despite lower levels of circulating catecholamines in the sheep. Kidney tubular function as measured by an improved ability to handle salt and water loads was also improved by fetal exposure to corticosteroids. A fetal exposure to corticosteroids resulted in an integrated response that made the preterm lamb more tolerant to postnatal asphyxia (11). The pleiotropic organ maturational effects of corticosteroids result in global adaptive responses that benefited the preterm. Within the context of normal development, these effects can be viewed as replicating the normal fetal adaptations to term birth.
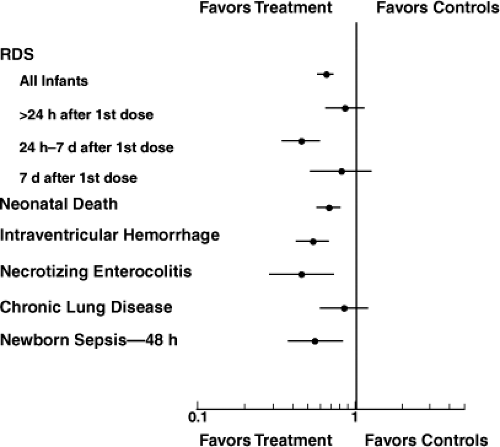 Figure 19.1. Meta-analysis of randomized controlled trials of antenatal corticosteroids. The point estimates for the risk ratios and 95% confidence intervals are the summary values for the composite analysis. Antenatal glucocorticoids improve outcomes for preterm infants. RDS, Respiratory distress syndrome. Based on data from Roberts and Dalziel (8). |
A continuing concern has been the potential for harm from hormones that can clearly alter development, cause dysmorphic changes in rodents, and decrease brain and body growth in multiple animal models (12). A single course of corticosteroids did not decrease birth weight in the preterm human in 11 studies of 3,586 infants (weight difference -17.5 g; 95% CI, -62 to 27 g). When used as a single treatment course at gestations more than 28 weeks, antenatal corticosteroids have been remarkably safe. No acute adverse effects after preterm birth have been reported despite widespread use. The risks of infection in the newborn and postpartum infection in the mother were not increased (8). The lingering concern has been potential long-term adverse effects of antenatal corticosteroid exposure of preterm fetuses. Long-term outcomes are available and are reassuring. Children exposed to a single course of antenatal corticosteroids were taller and had better cognitive function than controls at a 14-year follow-up (13). However, they also had higher blood pressures, although few were in the hypertensive range (14). In contrast, in 6-year and 23-year follow-up reports, the steroid exposed young adults had lower systolic blood pressures (15,16). A 30-year follow-up of the newborns from the original Liggins and Howie trial found some evidence of insulin resistance in the steroid-exposed adults, but no other cardiovascular abnormalities relative to controls (17). The remaining concern is corticosteroid effects of very early gestational exposures, which is not addressed by follow-up of children from the early trials.
Pharmacology and Dose of Antenatal Corticosteroids
The Consensus Conference recommended maternal treatment with betamethasone or dexamethasone rather than cortisol or methylprednisolone because the fluorinated corticosteroids cross the placenta from the mother to the fetus, have no mineralocorticoid activity, and have a relatively long duration of action (4). Betamethasone and dexamethasone are equivalent structurally except for the isomeric position of the 16-methyl group. Betamethasone was given as the suspension of relatively insoluble acetate and soluble phosphate by Liggins and Howie in the original trial (2), and that formulation,
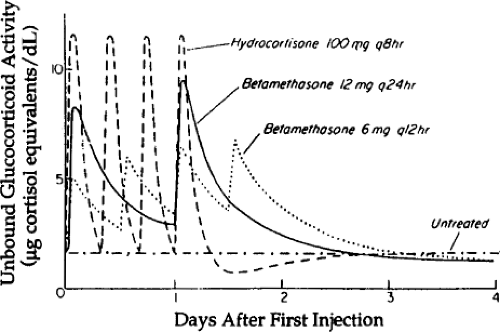
marketed in the United States as Celestone Soluspan® (Schering Plough) is the only betamethasone preparation available for injection. Betamethasone phosphate preparations are available elsewhere. Dexamethasone is given as the soluble sodium phosphate. The soluble phosphates of betamethasone and dexamethasone are prodrugs that are rapidly dephosphorylated to the active forms and have a rapid onset of action when given by intramuscular injection. Two dosing schedules were empirically evaluated in the clinical trials and are currently the recommended dosing schedules. A total dose of 24 mg betamethasone (acetate plus phosphate) is given as a divided dose of 12 mg at the recognition of risk of preterm delivery and 24 hours later. The same total dose of 24 mg of betamethasone sodium phosphate is given as a four-dose treatment, with 6 mg given at the recognition of the risk of preterm delivery and three subsequent doses of 6 mg given at 12-hour intervals. The resulting corticosteroid exposures for the fetus were modeled by Ballard and Ballard (18) (Fig. 19.2). Both agents readily cross the placenta and achieve fetal plasma levels of 30% to 40% of the maternal levels. There are isolated reports of higher corticosteroid doses or shorter treatment intervals to “accelerate” the maturation if delivery cannot be delayed for 48 hours, but no clinical data support these other dosing strategies.
marketed in the United States as Celestone Soluspan® (Schering Plough) is the only betamethasone preparation available for injection. Betamethasone phosphate preparations are available elsewhere. Dexamethasone is given as the soluble sodium phosphate. The soluble phosphates of betamethasone and dexamethasone are prodrugs that are rapidly dephosphorylated to the active forms and have a rapid onset of action when given by intramuscular injection. Two dosing schedules were empirically evaluated in the clinical trials and are currently the recommended dosing schedules. A total dose of 24 mg betamethasone (acetate plus phosphate) is given as a divided dose of 12 mg at the recognition of risk of preterm delivery and 24 hours later. The same total dose of 24 mg of betamethasone sodium phosphate is given as a four-dose treatment, with 6 mg given at the recognition of the risk of preterm delivery and three subsequent doses of 6 mg given at 12-hour intervals. The resulting corticosteroid exposures for the fetus were modeled by Ballard and Ballard (18) (Fig. 19.2). Both agents readily cross the placenta and achieve fetal plasma levels of 30% to 40% of the maternal levels. There are isolated reports of higher corticosteroid doses or shorter treatment intervals to “accelerate” the maturation if delivery cannot be delayed for 48 hours, but no clinical data support these other dosing strategies.
The pharmacology of corticosteroids for the indication of maternal corticosteroids for fetal lung maturation has not been well studied. The dosing schedules were selected quite empirically on the basis of what seemed to work in the initial trial (2). In fetal sheep models, single doses of dexamethasone phosphate or betamethasone phosphate given to the fetus or ewe do not induce lung maturation (19), nor do single fetal doses of cortisol (20). Repetitive dosing with the soluble phosphates or cortisol does induce lung maturation, suggesting that prolonged exposure is important to the response. In sheep, fetal treatments with betamethasone acetate plus phosphate induce some lung maturation, whereas maternal treatments induce more lung maturation and also fetal growth restriction (21). The maternal dosing results in much lower fetal blood levels of betamethasone but causes larger fetal effects. The slow release betamethasone acetate increases blood levels in the ewe minimally but will induce fetal lung maturation in sheep. Low and repeated maternal doses of dexamethasone also cause fetal growth restriction in sheep (22). These observations have not been exploited to develop the minimally effective dose and formulation of corticosteroid for clinical use.
Betamethasone versus Dexamethasone
Antenatal treatments with either agent decrease RDS and intraventricular hemorrhage (8), which means that comparisons of efficacy are evaluating differences in responses against a background of benefit. There are a number of reports comparing the efficacy of maternal treatments with betamethasone or dexamethasone and a recent meta-analysis of the randomized controlled trials (23). The relative efficacy of the treatments can also be compared indirectly by evaluating the responses of each treatment relative to controls using the larger data set from the Roberts and Dalziel meta-analysis (8) (Table 19.2). Either comparison of dexamethasone and betamethasone treatments demonstrates more intraventricular hemorrhage in the betamethasone-treated infants, similar fetal/neonatal deaths, and a better efficacy for betamethasone to decrease RDS than dexamethasone. The increased intraventricular hemorrhage results primarily from the large Elimian trial (24), which contributed about 80% to this outcome in the direct meta-analysis. There are no randomized and controlled data on longer-term outcomes. A recent observational study suggests better neurological outcomes at 2 years for betamethasone-exposed children than for dexamethasone-exposed children (25). Thus, the clinical benefits of one treatment choice versus the other are not compelling. The large trials of repeated courses of antenatal corticosteroids selected betamethasone as the treatment drug.
If these drugs are so similar, should there be any differences in clinical responses? The drug formulations and timing of dosing are different, so any differential clinical
responses may not result from the drugs themselves. However, there is physiologic information indicating that maternal/fetal responses to the treatments differ. (Table 19.2) In sheep, fetal treatment with betamethasone more predictably induced preterm labor than did dexamethasone (26). In mice, betamethasone was a more potent inducer of lung maturation and had less effect on subsequent neurodevelopment than did dexamethasone (27). Both betamethasone and dexamethasone altered fetal heart rates in humans, but betamethasone had less effect on fetal heart rate variability (28). Although the genomic potencies of betamethasone and dexamethasone are similar, these corticosteroids also have rapid effects on cell membrane functions such as ion transport that can alter intracellular signal transduction pathways (29). Corticosteroids can also modulate metabolic pathways that regulate endothelial nitric oxide synthase by nonnuclear effects (30). For some nongenomic effects, dexamethasone is much more potent than betamethasone (31). These drugs are not equivalent and the preferred drug for antenatal treatments remains unclear because of unknown differences in these drugs for this indication as well as formulation and dosing differences.
responses may not result from the drugs themselves. However, there is physiologic information indicating that maternal/fetal responses to the treatments differ. (Table 19.2) In sheep, fetal treatment with betamethasone more predictably induced preterm labor than did dexamethasone (26). In mice, betamethasone was a more potent inducer of lung maturation and had less effect on subsequent neurodevelopment than did dexamethasone (27). Both betamethasone and dexamethasone altered fetal heart rates in humans, but betamethasone had less effect on fetal heart rate variability (28). Although the genomic potencies of betamethasone and dexamethasone are similar, these corticosteroids also have rapid effects on cell membrane functions such as ion transport that can alter intracellular signal transduction pathways (29). Corticosteroids can also modulate metabolic pathways that regulate endothelial nitric oxide synthase by nonnuclear effects (30). For some nongenomic effects, dexamethasone is much more potent than betamethasone (31). These drugs are not equivalent and the preferred drug for antenatal treatments remains unclear because of unknown differences in these drugs for this indication as well as formulation and dosing differences.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree