EVIS SALA  YULIA LAKHMAN
YULIA LAKHMAN  WEINING MA
WEINING MA  HARPREET PANNU
HARPREET PANNU  DUAN LI
DUAN LI  NEETA PANDIT-TASKAR
NEETA PANDIT-TASKAR  HEDVIG HRICAK
HEDVIG HRICAK
INTRODUCTION
The objectives of imaging in gynecologic oncology are primary tumor detection and characterization, image-guided percutaneous biopsy, staging, monitoring treatment response, and detecting tumor recurrence. Comprehensive imaging of the female pelvis can be achieved using a combination of ultrasound (US), computed tomography (CT), magnetic resonance imaging (MRI), and combined 2-[18F]-fluoro-2-deoxy-D-glucose positron emission tomography computed tomography (FDG-PET/CT). The choice of imaging modality depends on the clinical question (Table 11.1). In some instances, more than one modality may be appropriate, and therefore it is best to discuss the clinical question with the radiologist to determine which modality will be most useful. As with any rapidly evolving technology, imaging strategies are not static and require continuous updating and reevaluation.
IMAGING MODALITIES, EXAMINATION TECHNIQUES, AND NORMAL ANATOMY
Ultrasound
Ultrasound (US) is considered the initial imaging modality of choice for evaluation of the female pelvis. Currently, the accepted roles of US in women with suspected gynecologic malignancy include characterization of adnexal masses, identification of endometrial abnormalities in women with intermenstrual or postmenopausal bleeding, and detection of primary or recurrent gestational trophoblastic disease in women with elevated serum beta human chorionic gonadotropin (β-hCG) levels. US is also used to guide biopsy of primary pelvic tumors and/or metastases (1) and to guide drainage of postoperative fluid collections such as lymphoceles, seromas, or abscesses. The role of US in screening high-risk patient populations for ovarian or endometrial carcinoma remains controversial.
US has many advantages in routine pelvic imaging: it is relatively inexpensive, provides multiplanar views, is widely available, and lacks ionizing radiation. Its portability allows it to be used in virtually any setting including the US suite, operating room, patient bedside, or radiation therapy suite. However, US also has a number of limitations: it is operator-dependent, and image quality varies with patient body habitus and presence of overlying bowel gas. Transvaginal ultrasound (TVS) provides the best spatial and soft-tissue resolution, but it comes at the cost of a significant decrease in the size of the field of view. Sonohysterogram (SHG), which utilizes TVS while distending the endometrial cavity with fluid, can be useful in evaluating the endometrium.
Technique
Adequate US evaluation of the female pelvis requires a transvaginal approach. Placement of the transducer closer to the organs of interest allows use of a higher-frequency (typically 5.0- to 7.5-MHz) transducer. While the use of such a transducer results in significantly improved spatial resolution and fewer imaging artifacts in comparison to transabdominal imaging, it does so at the cost of a smaller field of view. Hence, transabdominal imaging must be considered an important complementary imaging technique if the mass is larger than the TVS field of view, the ovaries are displaced out of the pelvis, the ovaries are not visualized because of the presence of bowel gas, or to evaluate important ancillary findings such as hydronephrosis, ascites, peritoneal deposits, and liver metastases. Transabdominal imaging also provides a better overall view of the pelvis and the relationship of female pelvic organs to the pelvic sidewall and bladder. Transabdominal pelvic imaging is performed with a 3.5- to 5.0-MHz curved array transducer using a distended urinary bladder as an acoustic window to view the female pelvic organs. Three-dimensional US is a relatively new technique that has been shown to decrease scanning time and possibly improve diagnostic confidence in pelvic imaging (2,3). Color, power, and spectral Doppler provide additional information regarding associated vascularity.
SHG is increasingly performed to improve the evaluation of the endometrium. With this technique, sterile saline is infused into the endometrial cavity under continuous TVS guidance. The sterile saline distends the endometrial cavity, separating the anterior and posterior endometrial layers and outlining the endometrial surface (4,5). Contraindications to SHG include hematometra and acute pelvic inflammatory disease. Three-dimensional SHG reduces operator dependence and length of examination time and has been reported to provide significant additional information in a majority of patients (6).
Normal Anatomy
The normal myometrium is homogeneous in echotexture and intermediate in echogenicity. Anechoic tubular structures separating the outer one-third from the inner two-thirds of the myometrium represent the arcuate vessels, which are best seen on color flow imaging. The internal os can be recognized as a slight constriction of the uterine contour.
The endometrium should be measured at the thickest part of the endometrial stripe in the uterine fundus on a midline sagittal image and, by convention, is always reported as a double-layer measurement. The normal endometrium is echogenic. However, the thickness and echotexture of the endometrium vary with the hormonal status of the patient. At the end of the menses, the endometrial stripe is highly echogenic and measures approximately 2 to 3 mm (Fig. 11.1). During the secretory stage, the endometrium becomes less echogenic and thickens, reaching a maximum of 8 mm (Fig. 11.1). Before ovulation, a trilaminar appearance of the endometrium has been described. The central thin echogenic line represents a reflective artifact and/or mucus and secretions between the anterior and posterior layers of the endometrium. Subjacent is the second layer, which is the relatively hypoechoic functionalis layer. The third layer is the surrounding deeper basalis layer, which remains echogenic. Following ovulation, the functionalis layer becomes thicker and more echogenic than during the proliferative phase, and the entire stripe may reach 15 mm in thickness and is more uniformly echogenic just prior to menstruation (7). In some patients, the innermost layer of myometrium is relatively hypoechoic compared to the immediately adjacent endometrium and is termed the subendometrial halo. The subendometrial halo is less commonly visualized in postmenopausal women. Neither the subendometrial halo nor fluid or debris within the endometrial cavity should be included in measurements of the endometrial stripe. At SHG, the endometrium should be homogeneous, symmetric, and regular without mass effect (Fig. 11.2). The sum of the width of the two layers should not exceed the maximum acceptable width for the patient’s hormonal status.
The Choice of Imaging Modality in Endometrial, Cervical, and Ovarian Cancer |
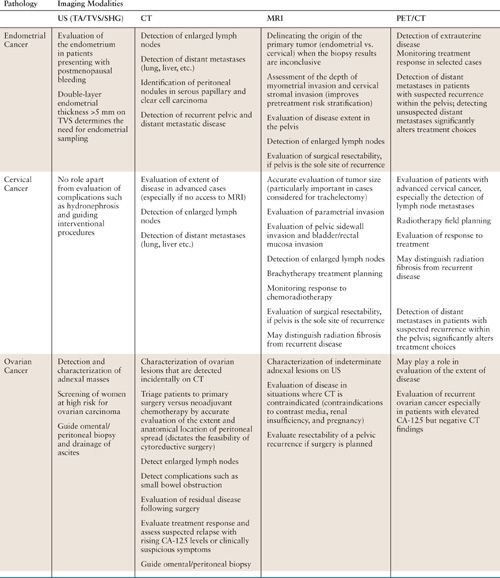
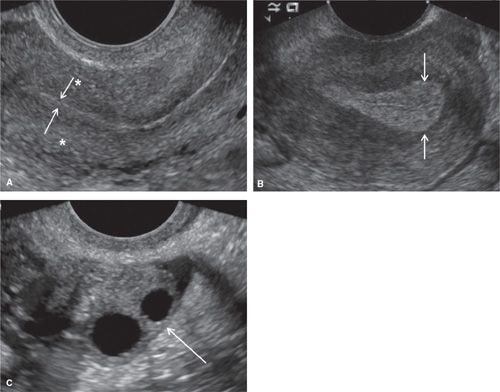
FIGURE 11.1. Normal uterus and ovary on ultrasound. Longitudinal TVS image (A) demonstrating normal uterine anatomy. Uterine myometrium demonstrates a homogeneous echotexture (* in A). The endometrium is a highly echogenic thin line (arrows in A) at the end of menstruation. Longitudinal TVS image (B) in a different patient demonstrates a normal endometrium (arrows in B) in the secretory phase. Transverse TVS image (C) demonstrates a normal ovary (arrow in C) in a premenopausal patient. Ovarian parenchyma demonstrates a homogeneous echotexture with multiple anechoic follicles.
In postmenopausal women, the uterine corpus atrophies until it approximates the length of the cervix. Similarly, there is atrophy of the endometrium which appears as a thin, regular echogenic line <5 mm in width (8). In women taking hormonal replacement therapy (HRT), less uterine and endometrial atrophy occurs (9). Women taking sequential HRT are best examined following withdrawal bleeding and the progesterone phase of their cycle when the endometrium is expected to be at the thinnest.
The US appearance of the ovaries also changes with the patient’s hormonal status. The premenopausal ovary has a relatively homogeneous outer cortex with a more echogenic central stroma. Small anechoic follicles are commonly seen peripherally (Fig. 11.1). At ovulation, one follicle becomes dominant, reaching a maximum diameter of 2.0 to 2.5 cm. Following ovulation, the corpus luteum develops. The corpus luteum may contain debris and/or hemorrhage and often involutes, appearing to be crenelated just prior to menstruation. The ovaries atrophy with menopause and contain fewer follicles. Therefore, postmenopausal ovaries are more difficult to visualize with US. In women taking HRT, less ovarian atrophy will occur and follicles will continue to develop.
Doppler evaluation of ovarian blood flow is best performed during days 3 to 7 of the menstrual cycle. The ovary undergoing ovulation in a given cycle demonstrates a relatively high-velocity, low-resistance arterial waveform with continuous forward diastolic flow. The contralateral ovary will typically demonstrate a higher-resistance, low-velocity arterial waveform with either very low or no diastolic flow.
Computed Tomography
CT is the primary imaging modality for staging and treatment follow-up of patients with ovarian cancer. It is also routinely used for detection of distant metastatic disease and lymphadenopathy in patients with advanced endometrial, cervical, and vulvar cancer as well as detection of recurrent gynecologic malignancies. CT rather than US-guided biopsy can be useful masses that are small and/or difficult to access (1,10–12).
Advantages of CT include ready availability, short image acquisition times, large field of view, high spatial resolution, and rapid three-dimensional (3-D) reconstructions. Disadvantages of CT include the use of ionizing radiation, degradation of image quality by patient body habitus or metallic implants, such as a hip prosthesis, and the morbidity and mortality associated with iodinated contrast agents.
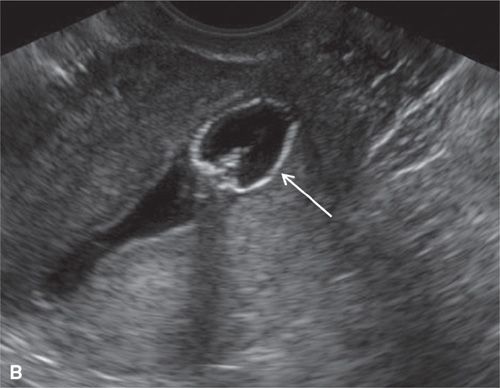
FIGURE 11.2. Normal sonohysterogram. Longitudinal SHG images (A, B) demonstrate a fluid-filled endometrial cavity (arrows in A). Catheter (arrows in B) is seen within the lower uterine segment.
Technique
Contrast-enhanced CT of the abdomen and pelvis is performed in the portal venous phase, 70 seconds following an injection of intravenous low osmolar contrast medium; this enhances blood vessels and viscera, allowing easier identification of enlarged lymph nodes and parenchymal lesions, especially in the liver and spleen. Oral contrast medium is utilized to opacify the small and large bowel, which allows detection of bowel serosal deposits. In some centers, patients receive 500 mL of diluted oral contrast medium the evening preceding the examination and an additional 500 mL, 45 minutes before the CT scan. In other protocols, the patient receives 600 to 1,000 mL of dilute oral contrast at least 1 hour before the examination, coupled with a 200-mL dilute contrast enema.
Normal Anatomy
On CT, the vagina, cervix, and uterine corpus can be differentiated based on both morphologic and enhancement characteristics (Fig. 11.3). The uterine corpus appears as a triangular or ovoid soft-tissue mass posterior to the urinary bladder. The cervix is a more rounded structure. At the level of the fornix, the vagina has a flat rectangular or crescentic shape. Following bolus intravenous contrast administration, the myometrium in the uterine corpus enhances to a greater degree than that of the cervix (Fig. 11.3). The endometrium can often be delineated from the enhancing myometrium. The broad and round ligaments can be seen coursing laterally and anteriorly, respectively. Occasionally, the uterosacral ligaments are depicted as arc-like structures extending from the cervix to the sacrum.
In the premenopausal patient, the normal ovaries are routinely seen, usually posterolateral to the uterine corpus and medial to the external iliac vessels (Fig. 11.3). Their uniform soft-tissue density is punctuated by small cystic regions representing follicles. In the postmenopausal patient, the ovaries are small and less readily detected.
Magnetic Resonance Imaging
MRI is the modality of choice for staging of uterine corpus and cervix malignancies. MRI has been shown to be superior to CT for staging of endometrial and cervical cancers (13,14), and may be useful for staging of ovarian cancer in selected patients. In addition, MRI can often differentiate radiation fibrosis from recurrent tumor (15) and is useful in the detection of recurrent gynecologic malignancies. The accuracy of MRI assessment of lymph node involvement is similar to that of CT; both rely on size criteria to detect lymphadenopathy (16).
Although MRI is still relatively expensive, it minimizes costs in some clinical settings by limiting or eliminating the need for more expensive and/or more invasive diagnostic or surgical procedures (17,18). There are at least three cost-minimizing indications for MRI in the evaluation of women with gynecologic malignancy: (a) staging of invasive cervical carcinoma as an adjunct to clinical examination; (b) the preoperative management of women with endometrial cancer (19–21); and (c) the characterization of indeterminate adnexal lesions on US (18,22).
Advantages of MRI include superior soft-tissue contrast, absence of ionizing radiation, and multiplanar capability. MRI is the modality of choice for patients with allergies to iodinated intravenous contrast media or renal impairment. However, clinicians should be aware that gadolinium contrast agents used in MRI have recently been associated with the development of nephrogenic systemic fibrosis (NSF), a rare progressive connective tissue disease, in patients with end-stage renal disease and/or acute renal failure. One disadvantage of MRI relative to CT is the need for longer acquisition times. In addition, MRI is contraindicated in patients with pacemakers, cochlear implants, certain vascular clips, metallic objects in the eye, and neural stimulators. Furthermore, some patients may experience claustrophobia and may require sedation in order to complete a diagnostic examination.
Technique
Patients are asked to fast for 4-6 hours prior to MRI examination in order to limit motion artifacts from bowel peristalsis (23). An antiperistaltic agent, such as glucagon or hyoscine butyl bromide, is recommended to further reduce motion artifacts (24). The patient is asked to void before the examination, as a full bladder may cause ghosting and motion artifacts that degrade images. Patients are imaged in the supine position using a pelvic surface array multichannel coil (e.g., a cardiac coil usually offers the best image quality) (23,25). Endoluminal coils (endorectal and endovaginal) can provide high-resolution images of small tumors of the cervix and aid detection of early parametrial invasion (26,27). However, their field of view is limited in assessing large tumors, extra-uterine extension to adjacent organs and the pelvic sidewall, and detecting pelvic lymph nodes; consequently, endoluminal coils are not widely used in gynecological imaging. Vaginal opacification with gel, yielding high signal intensity on T2-weighted images (T2WI), may be useful in cases where there is suspected cervical tumor extension into the vagina, particularly into the posterior vaginal fornix (28).
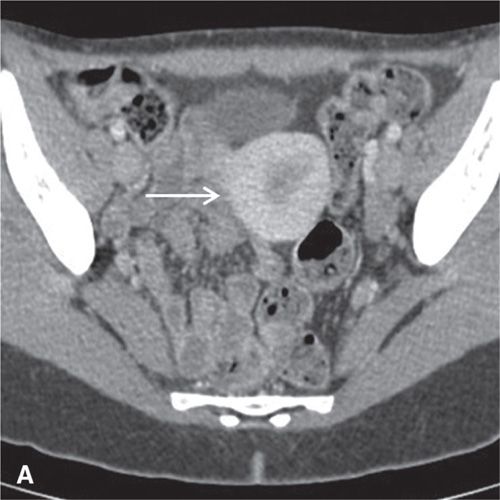
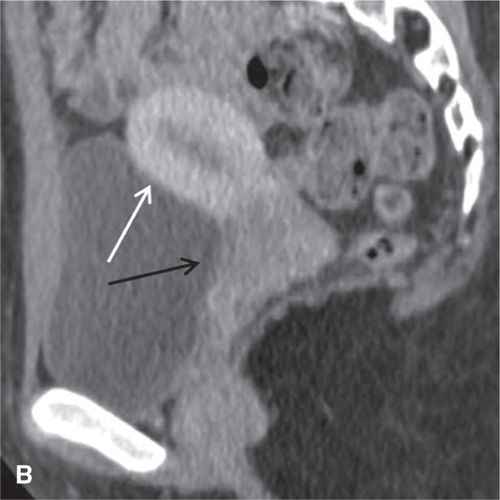
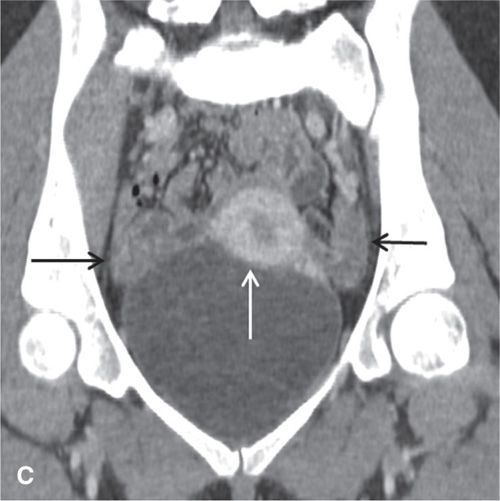
FIGURE 11.3. Normal uterus and ovary on CT. Axial contrast-enhanced CT image (A), sagittal (B), and coronal reformatted (C) CT images demonstrate a normal uterus (white arrow in A, B, C) and ovaries (black arrows in C). Note that the cervix (black arrow in B) enhances less avidly than the myometrium (white arrow in B); this should not be mistaken for cervical pathology. Both ovaries (black arrows in C) are seen as soft-tissue attenuation ovoid structures adjacent to the uterus. They contain small cystic spaces corresponding to follicles.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree