Jill Newstead-Angel, MD, FRCPC
George R. Saade, MD
Luis D. Pacheco, MD
2. Intermediate-Acting Insulin
3. Long-Acting Insulin (Detemir)
4. Long-Acting Insulin (Glargine)
• Limitations of Current Postpartum Testing Recommendations
• GDM: Intermediate/Late Testing
PREGESTATIONAL DIABETES
The hallmark of management for women with diabetes mellitus (DM) Type 1 or 2 is pre-pregnancy planning to educate/reinforce the concepts of a proper diet, understand the risks of uncontrolled diabetes before and during pregnancy, and optimize insulin management to achieve proper glycemic control starting as early as possible. The initial study correlating an elevated maternal HbA1c at the time of conception with major congenital fetal malformations found that the risk is approximately doubled when the value is >7% and increased four to five times when the value is ≥8.6%.1 Subsequent studies have found that the relative risk goes up when the HbA1c is ≥6.6%, and a systematic review noted that pre-pregnancy care can reduce the HbA1c by 1.9%.2,3 Clinicians should recognize maternal and obstetric issues that identify women at risk of future complications that require immediate forethought and planning on the part of the caregivers, and therefore, “interpregnancy care” begins immediately following delivery.
This proactive planning is intended to engage patients in the risk reduction care plan as early as possible. Because gestational DM (GDM) is a risk factor for Type 2 DM, women with a past history of GDM are the perfect target for this kind of planning as the prevalence of GDM doubled from 2% to 4% in the United States between 1994 and 2002 and tripled in Australia from 2.9% to 8.8% between 1971 and 1994.4–7 Identification and treatment of women with persistent postpartum dysglycemia have the potential to decrease hyperglycemia-associated teratogenesis and pregnancy loss in future pregnancies.
PRE-PREGNANCY CARE PLAN
As with any patient, diabetics should have a complete history and physical examination. Usual laboratory evaluation includes a baseline renal function (BUN and creatinine) and a 24-hour urine collection to quantify baseline proteinuria. Normal 24-hour protein excretion is <150 mg/24 hours in nonpregnant women and <300 mg/day during pregnancy. Abnormal excretion of protein is associated with diabetic nephropathy and increases the risk of developing preeclampsia. A spot protein/creatinine ratio may also be used to quantify proteinuria, but it is not as sensitive as a 24-hour collection. The initial clinical exam should include the following elements.
History
• Age of onset and duration of diabetes
• Classification into Type 1 or 2
• History of diabetic ketoacidosis (DKA)
• History of severe hypoglycemia
• Ability to develop symptoms of hypoglycemia
• Retinopathy and/or treatment
• Proteinuria/renal function
• Hypertension
• Neuropathy
• Cardiac disease (obtain electrocardiogram)
• Cerebrovascular disease.
Physical Exam
• Height, weight, BMI calculation, blood pressure
• General exam including assessment of peripheral edema
• Reflexes
• Referral for retinal exam
• Fundoscopic examination.
Labs
• HbA1c
• 24-hour urine collection for proteinuria
• Complete blood count
• Metabolic panel
• Thyroid-stimulating hormone (in Type I DM)
• Echocardiogram not routinely indicated.
When feasible, pregnancy should be delayed until adequate glucose control is achieved with HbA1c levels ideally within the normal range (less than 5.5%). Pregnancy should be avoided in the setting of elevated glycosylated hemoglobin, as a direct relationship exists between congenital malformations and elevated glucose levels. Women with Type 2 diabetes on oral medications should ideally be transitioned to insulin, as the data on the use of oral agents for pregestational diabetes during pregnancy are very limited.
Diabetic nephropathy is characterized by glomerular nodular sclerosis and commonly manifests as nephrotic syndrome. Pregnancy by itself does not alter the natural evolution of the nephropathy. Patients with nephrotic syndrome and hypertension are commonly managed with angiotensin converting enzyme inhibitors or angiotensin II receptor blockers as these agents dilate preferentially the efferent arteriole in the nephron resulting in decreased glomerular filtration pressure and decreased proteinuria. The latter agents are contraindicated in pregnancy and patients should be transitioned to alternative antihypertensive agents such as labetalol. Calcium channel blockers (such as nifedipine) may be used; however, they may dilate the afferent arteriole resulting in increased proteinuria. In the setting of end organ damage, such as diabetic nephropathy, blood pressures should be maintained close to 130/80.
Evaluation of the retina is recommended before pregnancy. Background retinopathy (nonproliferative) does not require treatment before pregnancy other than optimization of glucose control. If proliferative retinopathy is diagnosed before pregnancy, referral to an ophthalmologist is mandatory as laser treatment will be required. Noncorrected proliferative nephropathy may worsen during pregnancy and is a contraindication for a vaginal delivery as hemorrhage and retinal detachment from subsequent fibrosis may result in permanent blindness.
A nutritional consultation to review/reinforce the dietary requirements during the upcoming pregnancy is also advised. Women need to understand that insulin requirements will change during pregnancy. Lean women with type I diabetes may need less insulin during the first half of pregnancy as estrogen-induced increase in insulin production by beta cells will result in increased insulin levels at an early period when significant levels of human placental lactogen (placental origin) have not been reached. Similarly, as pregnancy progresses and levels of human placental lactogen increase, insulin requirements will increase as insulin resistance progresses.
Overweight and obese women should work toward a BMI <25 kg/m2 to decrease the risks of stillbirth, preeclampsia, macrosomia, and peripartum infections. Classification of diabetes during pregnancy is depicted in Table 13-1.
Diabetes Classification During Pregnancy |

MANAGEMENT IN PREGNANCY
As discussed previously, women with pregestational diabetes will require insulin to achieve adequate glucose control during pregnancy as minimal data exist on the use of oral hypoglycemic agents in this group. Anticipate that insulin requirements will increase as pregnancy progresses.8
Titration of insulin doses should be aggressively pursued so that fingerstick glucose values are in line with those recommended by the American Diabetes Association (ADA) and the American College of Obstetricians and Gynecologists during pregnancy: fasting blood glucose concentration ≤95 mg/dL (5.3 mmol/L); 1-hour postprandial blood glucose concentration ≤140 mg/dL (7.8 mmol/L); 2-hour postprandial glucose concentration ≤120 mg/dL (6.7 mmol/L).9,10
During pregnancy, data support monitoring fasting and 2-hour postprandial glucose values as it can improve glycemic control and decrease obstetric complications (eg, fetal macrosomia) compared with typical preprandial monitoring. A small-randomized clinical trial (n = 61) found that postprandial monitoring achieved glycemic control in 55% versus 30% using preprandial monitoring. Similarly, patients monitored using postprandial values had less preeclampsia.11 A study in 66 women with GDM found that postprandial monitoring was associated with lower birth weights and less neonatal hypoglycemia (3% postprandial group vs 21% preprandial group).12 In summary, glucose control during pregnancy should be monitored measuring fasting and 2-hour postprandial glucose values and insulin should be adjusted based on such values.
Choice of Insulin Regimen
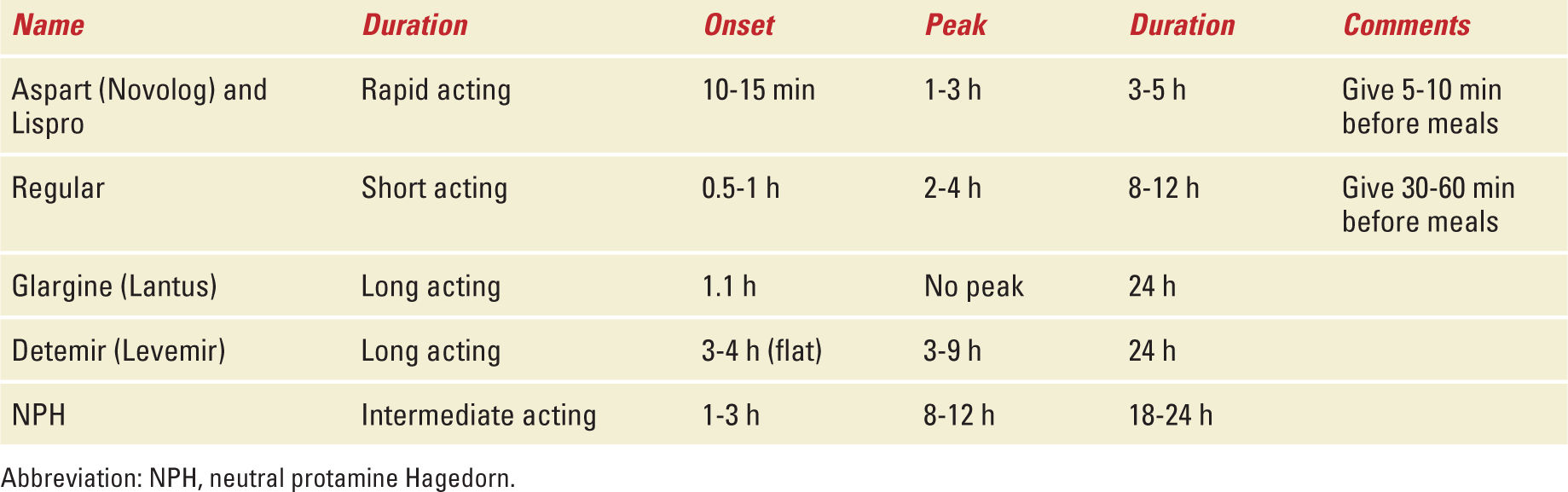
Classically, management during pregnancy requires a weight-based regimen consisting of a combination of regular and neutral protamine Hagedorn (NPH) insulins. The initial daily dose is 0.8 U/kg/day in the first trimester, 1 U/kg/day in the second trimester, and 1.2 U/kg/day during the third trimester. The total dose is divided so that 2/3 is given in AM (30-60 minutes before breakfast) and the other 1/3 in PM (30-60 minutes before dinner). From the total AM dose, 2/3 is given in the form of NPH and the other 1/3 as regular insulin. The PM dose is divided so that half is regular insulin and the other half is NPH. Regular insulin peaks at 2 to 4 hours after administration, consequently regular insulin administered before breakfast and dinner will control the 2-hour postprandial values after such meals. NPH peaks at 7 to 8 hours after administration; the prebreakfast dose of NPH will control the glucose value 2 hours after lunch and the predinner NPH dose is expected to control fasting glucose values. Patients who present with elevated fasting values (above 95 mg/dL) should be evaluated carefully. A glucose measurement at 3 AM is recommended to rule out the possibility of the Somogyi effect. If the glucose value at 3 AM is low (less than 60 mg/dL), the body will secrete counter regulatory hormones (eg, cortisol, glucagon, catecholamines) resulting in elevated serum glucose in the morning hours. The latter is the Somogyi effect and requires a decrease in the NPH administered at night. Another option is to give the PM NPH at bedtime (eg, 10-11 PM) as opposed to predinner so that the peak of action of NPH will occur at 6 to 7 AM when the patient is awake and if hypoglycemia occurs, she can eat a snack. Pregnant women are more prone to hypoglycemia at nighttime as the placenta and fetus continue to consume glucose overnight when the patient is not eating. If the 3 AM measurement is normal, then the fasting hyperglycemia is corrected by increasing the PM NPH dose. NPH and regular insulin have been around the longest and therefore have the most data surrounding their use; however, data exist regarding the safety of new long-acting (Detemir) and short-acting analogues (aspart/lispro).
Occasionally, patients may be treated with continuous subcutaneous insulin pumps.13 There is no good evidence to suggest that a pump is superior to a well-designed split dose regimen as discussed earlier.
Rapid-Acting Insulins
Aspart and lispro may be used during pregnancy. The advantage of these forms is that the onset of action is approximately 5 minutes (as opposed to 30 minutes for regular insulin) with a peak at 1 hour (as opposed to 2-4 hours for regular insulin) leading to a lower risk of postprandial hypoglycemia. There is less data regarding glulisine use during pregnancy. A retrospective review of 533 pregnancies in women using aspart found no increase in the incidence of congenital abnormalities compared with historical controls.14 A small series suggests that aspart and lispro are not inferior to human regular insulin, but the numbers were too small to draw any conclusions about superiority.15 A randomized study of 322 women reported no difference in obstetric outcomes when women used regular insulin versus aspart for the bolus doses.16 All women used concomitant NPH.
Intermediate-Acting Insulin
NPH is the historical standard and intermediate insulin of choice for use during pregnancy. Premixed formulations of 70/30 (70% NPH and 30% regular) do not provide physiological coverage and are reserved for patients who are unable or refuse to use other more appropriate regimens.
Long-Acting Insulin (Detemir)
Limited data exist regarding the use of detemir, two randomized studies of pregnant women found insulin detemir to be noninferior to NPH.17,18
Long-Acting Insulin (Glargine)
A retrospective review of 107 pregnancies among women using glargine before pregnancy found that glycemic control, birth weight, and the prevalence of macrosomia and neonatal morbidity were similar among the group that stopped it during the first trimester versus the group that continued it throughout pregnancy.19 A retrospective review of 67 women on insulin detemir and 46 on glargine found the prevalence of large for gestational age (LGA) infants lower with glargine 14 (30%) versus 33 (49%) with detemir. The mean HbA1c was 6.2% in the first and third trimesters for both groups.20 A systematic review of 1000 pregnancies during which glargine was used concluded that “Use of insulin glargine during pregnancy should be seriously considered in uncontrolled diabetes and in those patients taking insulin glargine before conception because the benefits from improved glycemic control would be expected to outweigh any, as yet, unproven risks of insulin glargine exposure.”21 An in vitro study concluded that typical therapeutic doses of glargine are not likely to cross the placenta.22 Glargine has an onset of action of 1 hour, no peak activity, and duration of 24 hours. It is usually administered once a day. There is no evidence that glargine is superior to the traditional split regimen (using NPH and regular insulin) described earlier.
Given the existing data from case series and small trials, it is reasonable to conclude that the primary goal should be glycemic control, and the insulin regimen used should be the one with which the patient is most likely to be compliant. In cases where access/insurance issues would make the cost of particular insulin prohibitive, then clinical judgment should prevail with the goal being glycemic control using the best available option. There is no data that any of the available insulins pose a tangible risk to the fetus. Table 13-2 summarizes the characteristics of different insulin preparations.
Insulin Pharmacodynamics |
GESTATIONAL DIABETES
GDM is defined as glucose intolerance with onset or first recognition during pregnancy. Approximately 7% (range 1%-14%) of all pregnancies are complicated by GDM, leading to about 200,000 cases/year in the United States.23 Immediately after delivery 20%-30% of these women have persistent dysglycemia (Type 2 DM or prediabetes [pre-DM]). As mentioned earlier, this at-risk population is increasing as the prevalence of GDM is increasing worldwide.4–7,24 There is evidence that treating GDM can decrease the risk of preeclampsia, cesarean section, LGA babies (>4000 g), and shoulder dystocia.25
Screening and Diagnosis
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


