Developmental Toxicology
Donald R. Mattison
Introduction
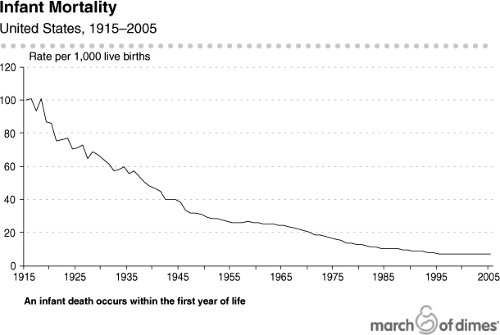
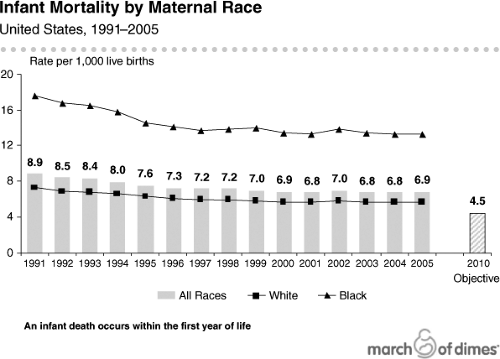
The United States has made progress in maternal and infant health (1,2,3,4); infant mortality has fallen (Fig. 11.1) as a consequence of pediatric nutrition, enhanced sanitation and food safety, and improvements in care for premature infants (5,6,7,8,9,10,11,12). Despite these advances, the United States has the worst rate of infant mortality among industrialized nations and ranks 30th internationally (Table 11.1) (12,13,14). In addition, substantial disparities in infant mortality have persisted despite attempts to alleviate these differences (Fig. 11.2) (15,16,17,18,19,20).
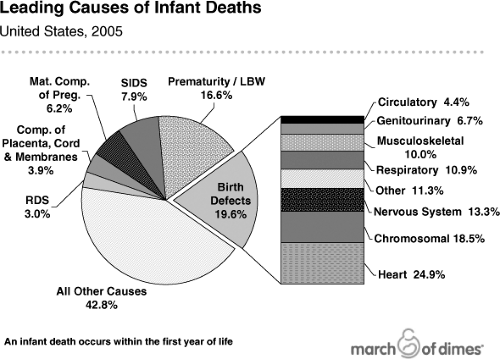
As a consequence of the decrease in overall infant mortality, the leading cause of infant death in the United States and in many developed countries today is birth defects (21,22,23), which account for ∼25% of all infant deaths (Fig. 11.3). Prematurity and its consequences, including low birth weight and respiratory distress syndrome, is the second leading cause of infant mortality overall. Among African Americans, prematurity is the primary cause of infant mortality, accounting in part for the substantially higher infant mortality rates in that community.
The increasing relative impact of birth defects on infant mortality is drawing more attention to this public health problem. For example, there is mounting concern about environmental pollutants and their impact on pregnancy outcome and infant health (24,25). Similarly, there is growing interest in the effect of disasters (Katrina, Ike, and World Trade Center) on pregnancy outcome and infant health (26). The Trust for America’s Health has mounted a campaign to critically evaluate the ability of state health departments to monitor birth defects and use the data in public health decision making (27). The Birth Defects Prevention Act of 1998 (Public Law 105-168) authorized the Centers for Disease Control and Prevention (CDC) to collect, analyze, and make available data on birth defects. The Children’s Health Act of 2000 established a National Center on Birth Defects and Developmental Disabilities (NCBDDD) at the CDC (28). The CDC provides funding to address problems that hinder birth defect surveillance programs (29) which are critical to identification of causes and interventions. Unfortunately, the causes of many birth defects are poorly understood (30,31,32,33). When the cause of a particular birth defect is unknown, health scientists suspect the possibility that environmental or occupational exposures may play a substantial role (31,32,33,34,35,36).
This chapter summarizes knowledge about (a) causes of birth defects and other adverse developmental outcomes (including death and abnormalities of function or growth); (b) levels of exposure to potential developmental hazards; (c) ways to determine whether an exposure to a chemical, physical, or a biological agent may be capable of producing embryo or fetal death, structural malformations, functional abnormalities, or alterations in growth; and (d) the level of certainty (or uncertainty) with regard to various data and risk assessments (37).
Background on Developmental Disease
Developmental diseases may be described in terms of origins or outcomes. Table 11.2 provides definitions of terms associated with developmental diseases.
Origins of Developmental Disease
Some birth defects occur because an embryo or fetus is destined to develop abnormally from the time of fertilization (36,37,38). These are called malformations. The risk of developing a malformation sometimes can be reduced. For example, folic acid supplements (400 μg) taken prior to fertilization (conception) may reduce the risk of neural tube defects, a serious and common malformation (39,40,41,42,43,44,45), by as much as 70% (35,42,43,44,45). Of interest are recent data suggesting that increasing the dose (up to 4 mg) may further reduce the risk of developmental disease (42,43,44,45). Note, however, that not all neural tube defects result from deficiencies in folic acid (46,47,48,49).
Other developmental diseases occur because fetal growth is physically restricted (deformation) (50). An infant with a deformation would have been normal if the physical restriction of growth had not occurred during intrauterine development (51,52). One example of a deformation is Potter syndrome (53), a deformation produced by inadequate formation of amniotic fluid, which results in abnormal skeletal and lung development (54,55,56,57).
An infant who might have been normal at birth but instead is born with a developmental disease because of
exposure to a chemical, physical, or a biological agent has a preventable developmental disease called a disruption. Examples of disruptions include congenital rubella syndrome, neurodevelopmental abnormalities produced by exposure to lead, and those produced by exposures to ionizing radiation (31,32,36,58,59,60).
exposure to a chemical, physical, or a biological agent has a preventable developmental disease called a disruption. Examples of disruptions include congenital rubella syndrome, neurodevelopmental abnormalities produced by exposure to lead, and those produced by exposures to ionizing radiation (31,32,36,58,59,60).
Table 11.1 Comparison of Infant Mortality Rates (Deaths/1,000 Live Births) and International Rankings for Selected Countries | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||
Table 11.2 Working Definitions of Terms Used in this Chapter | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Estimates of the percentage of birth defects that might be related to environmental risks have evolved over time as scientists’ understanding of developmental processes and causes of disease have progressed (Table 11.3). However, all estimates are problematic when one considers that many birth defects may have more than one cause. Earliest estimates (30,58,61) suggested that about 10% of birth defects were due to teratogens, 25% were due to genetic/chromosomal abnormalities, and 65% were of unknown origin. These estimates were widely cited in most publications about the origin of developmental abnormalities until a 1989 study by Nelson and Holmes (62) estimated that about 3% of birth defects are due to teratogens, 17% are genetic/chromosomal, 37% are due to gene–environment interactions, and 43% are of unknown origin. Each of these estimates was consistent with the scientific understanding at the time, which suggested that single factors were important causal agents (e.g., infectious agents, physical factors, chemicals, or maternal conditions responsible for most birth defects.
Table 11.3 Estimates of the Environmental Impact on Birth Defects | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
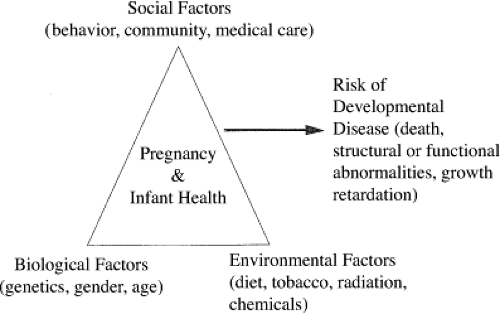
Our current scientific understanding has evolved. Thus, while earlier estimates of etiology or causation of developmental abnormalities emphasized the small number of birth defects caused by known teratogens (less than 10%), more recent estimates have included an increasing percentage of birth defects produced by gene–environment interactions (62,63). Gene–environment interactions refer to the fact that the individual carries genetic factors that modify the susceptibility of the fetus to the disruptive effect of an environmental agent that produces the birth defect or developmental abnormality observed (24,64,65,66). This leads to a model for developmental disease like that illustrated in Figure 11.4, in which the risk for a developmental disease is a consequence of an interaction among environmental, social, and biological factors (67,68,69). G. Shaw (oral communication, 2000), using data from the California Birth Defects Monitoring system, estimated that many birth defects (up to 75%) are due to gene–environment interactions. In contrast, estimates of structural malformations of unknown origin have decreased from 65% to 43% to 25%, suggesting an increase in knowledge. However, there remains much uncertainty about the diseases thought to be caused through gene–environment interaction.
Why do scientists think environmental factors play any role in birth defects? Studies have demonstrated that animals treated with chemicals found in the environment and for which there is human exposure produce birth defects in the animals; human epidemiologic studies have also demonstrated an association between such exposures and developmental disease (31,32,36,60,70). In addition, epidemiologists have explored the relationship between the environment and birth defects, with results that suggest that the environment appears to play a significant but poorly defined role, in part due to inadequate investment in biomarker-based epidemiologic research.
Outcomes
At least four health outcomes result from birth defects: death, structural abnormalities, functional abnormalities, and alteration of growth. A fifth outcome, premature birth, is also discussed briefly, although it is not usually categorized as a birth defect. Each type of outcome is illustrated in the following discussion.
Death
One class of drugs used to treat hypertension acts by inhibiting the angiotensin-converting enzyme (ACE). When experimental animals are treated with ACE inhibitors, fetal death rates increase (31,32,36,38,71,72). Similar observations have been made in women treated with ACE inhibitors during pregnancy (73,74). It is thought that fetal death results from a substantial reduction in renal blood flow and inhibition of renal development produced by these drugs. Because of the impact on fetal renal blood flow, despite the potential benefit for management of hypertension in some pregnant women, it is recommended that ACE inhibitors not be used during pregnancy.
Structural Malformation
It has been estimated that there are approximately 75 chemicals and drugs that are known to produce human
structural malformations (31,32,36,38,63,71,72). One well-known chemical that is associated with structural malformations of both male and female reproductive systems is diethylstilbestrol (DES). DES was used to treat women early in pregnancy because it was thought that it would prevent miscarriage and other pregnancy complications. Unfortunately, not only was DES unable to prevent pregnancy complications, it produced malformations of the male and female reproductive systems and increased the risk for vaginal cancer. Some investigators believe that the structural malformations were unlikely to have been identified if a randomized clinical trial had not been conducted to determine the therapeutic effectiveness of DES.
structural malformations (31,32,36,38,63,71,72). One well-known chemical that is associated with structural malformations of both male and female reproductive systems is diethylstilbestrol (DES). DES was used to treat women early in pregnancy because it was thought that it would prevent miscarriage and other pregnancy complications. Unfortunately, not only was DES unable to prevent pregnancy complications, it produced malformations of the male and female reproductive systems and increased the risk for vaginal cancer. Some investigators believe that the structural malformations were unlikely to have been identified if a randomized clinical trial had not been conducted to determine the therapeutic effectiveness of DES.
Functional Abnormality
Over the last three decades, there has been increased recognition that in addition to structural malformations produced by agents that are developmental hazards, functional abnormalities may also be produced. Areas of concern include intelligence, behavior, and performance of various tissues, organs, and systems. Prevention of functional abnormalities will require understanding of exposures known to modify the functional parameter being studied. One clear example is lead, which has been demonstrated cause lowered intelligence and produce behavioral abnormalities (31,32,36,38,63,71,72).
Growth
Growth, including birth weight and rate of weight gain after birth, appears to be a sensitive indicator of various insults during pregnancy and early postnatal development. While measures of growth are thought to be sensitive, they are not specific to chemical exposures because many different factors can influence growth both before and after birth. Prenatal exposure to tobacco smoke and alcohol are known to decrease fetal growth and increase the incidence of low birth weight (31,32,36,38,63,71,72).
Prematurity
Although premature birth is not traditionally included in the spectrum of adverse outcomes considered as developmental toxicology, there is growing evidence that it should be included (75). Premature birth is the second leading cause of death during the first year of life, and there is mounting evidence that environmental and social exposures may play a role in premature delivery (24,76,77). For example, it is known that smoking is associated with premature birth, some agricultural chemicals have been suggested to decrease the length of gestation, and it has been suggested that there are potential interactions between minor variations in the structure of genes (polymorphisms) and length of gestation (78,79,80,81,82). Data from Wang and her coworkers suggest that the length of gestation is decreased because of interactions between polymorphisms in genes involved in metabolism (cytochrome P450 and glutathione S-transferase) and benzene exposures that are below the permissible exposure limit set by the Occupational Safety and Health Administration. In addition, there is mounting evidence that air pollution may also play a role in prematurity (83).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree