8 Dermatology
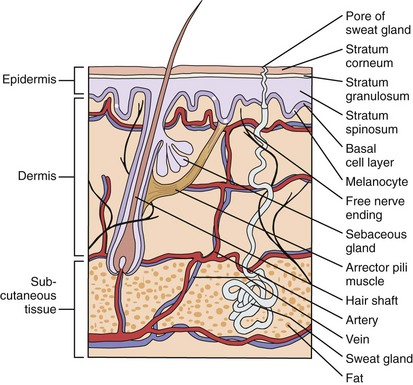
Most of us think of our skin as a simple, durable covering for our skeleton, muscles, and internal organs. However, the skin is a complex organ, consisting of many parts and appendages (Fig. 8-1). The outermost layer, the stratum corneum, is an effective barrier to irritants, toxins, and organisms, as well as a membrane that holds in body fluids. The remainder of the epidermis manufactures this protective layer. Melanocytes within the epidermis help protect us from the harmful effects of ultraviolet light, and Langerhans cells are one of the body’s first lines of immunologic defense.
Examination and Assessment of the Skin
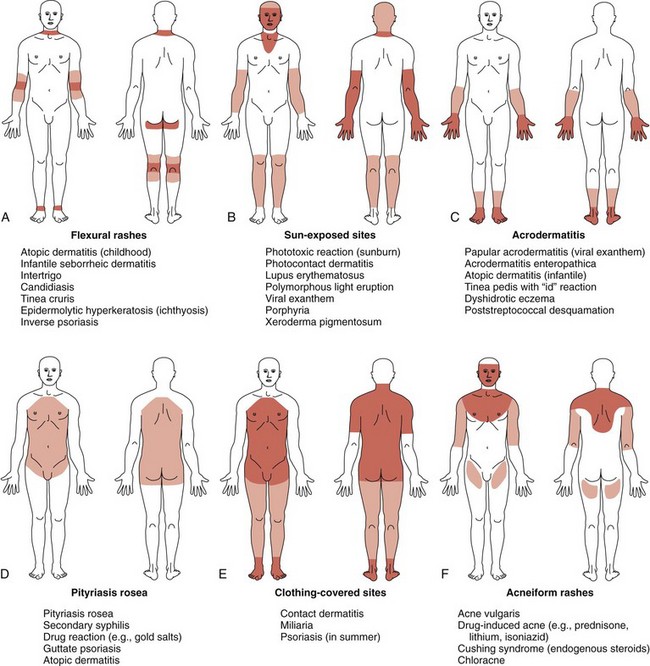
Despite the myriad conditions affecting the skin, a systematic approach to the evaluation of a rash or exanthem assists and simplifies the process of developing a manageable differential diagnosis. After assessing the patient’s general health, the practitioner should obtain a detailed history of the skin symptoms including the date of onset, inciting factors, evolution of lesions, and presence or absence of pruritus and/or fever, as well as any other systemic symptoms. Recent immunizations, infections, drugs, and allergies may be directly related to new rashes. The family history may suggest a hereditary or contagious process, and the clinician may need to examine other members of the family. Review of nursery records and photographs helps to document the presence of congenital lesions. Attention should then turn to the distribution and pattern of the rash. The term distribution refers to the location of the skin findings, whereas the term pattern defines a specific anatomic or physiologic arrangement (e.g., the distribution of a rash may include the extremities, face, or trunk, and the pattern could be flexural or intertriginous). Identification of a pattern can assist in the development of a differential diagnosis even before the detailed morphology of the skin lesions is studied. Other common patterns include photodistributed (involving sun-exposed sites), acral (involving primarily the distal extremities), and dermatomal configurations (Fig. 8-2).
Last, the practitioner should identify the morphology of the cutaneous lesions. Primary lesions (macules, papules, pustules, wheals, plaques, vesicles, bullae, nodules, and tumors) arise de novo in the skin. Secondary lesions (erosions, ulcers, crusts, excoriations, fissures, lichenification, atrophy, and scars) evolve from primary lesions or result from the patient’s manipulation (e.g., scratching, picking, or popping) of primary lesions. Delineation of the primary and secondary lesions allows the clinician to develop a differential diagnosis on the basis of the anatomic level of the skin lesions (Table 8-1). Disorders restricted to the epidermis may be associated with macular color changes, such as in vascular telangiectasias, freckles, and vitiligo. In epidermal disorders, surface markings are commonly altered by scales, vesicles, pustules, crusts, and erosions. Bullous impetigo, atopic dermatitis, and ichthyosis are primarily epidermal disorders. When the dermis is also involved, lesions usually display distinct borders because of dermal inflammation and edema. Disorders with both epidermal and dermal changes include psoriasis, lichen planus, and erythema multiforme. Inflammatory disorders or tumors restricted to the dermis do not usually alter the surface markings. Lesional borders are distinct, and color changes and edema may be present. Examples of dermal disorders include granuloma annulare, intradermal nevi, urticaria, and hemangiomas. The diagnosis of subcutaneous disorders is made by careful palpation. The surface markings are normal, and the color of the skin may be normal or red. There is altered skin firmness, and tenderness may be present. Subcutaneous lesions include lipomas, deep hemangiomas, hematomas, subcutaneous fat necrosis, and erythema nodosum.
Table 8-1 Anatomic Depth of Lesions
| Cutaneous Structure | Physical Findings | Specific Skin Disorders |
|---|---|---|
| Epidermis | Altered surface markings | Impetigo |
| Scale, vesicle, crust | Café-au-lait spot | |
| Color changes (black, brown, white) | Atopic dermatitis | |
| Vitiligo | ||
| Freckle | ||
| Epidermis and dermis | Altered surface markings | Psoriasis |
| Scale, vesicle, crust | Atopic dermatitis | |
| Distinct borders | Contact dermatitis | |
| Color changes (black, brown, white, and/or red) | Cutaneous lupus erythematosus | |
| Edema | ||
| Dermis | Normal surface markings | Urticaria |
| Color changes | Granuloma annulare | |
| Altered dermal firmness | Hemangioma | |
| Blue nevus | ||
| Subcutaneous tissue | Normal surface markings | Hematoma |
| Normal or red skin color | Cold panniculitis | |
| Altered skin firmness | Erythema nodosum |
From Cohen BA: Pediatric dermatology, ed 2, London, 1999, Mosby.
Papulosquamous Disorders
Psoriasis
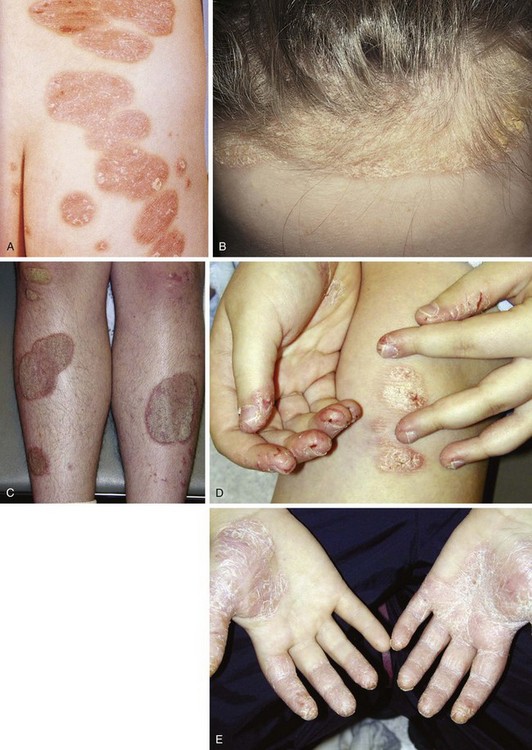
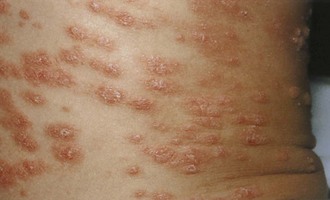
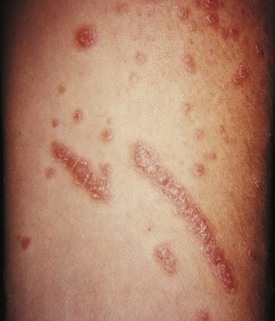
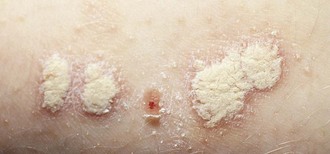
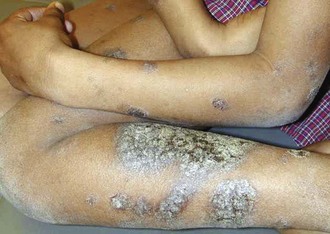
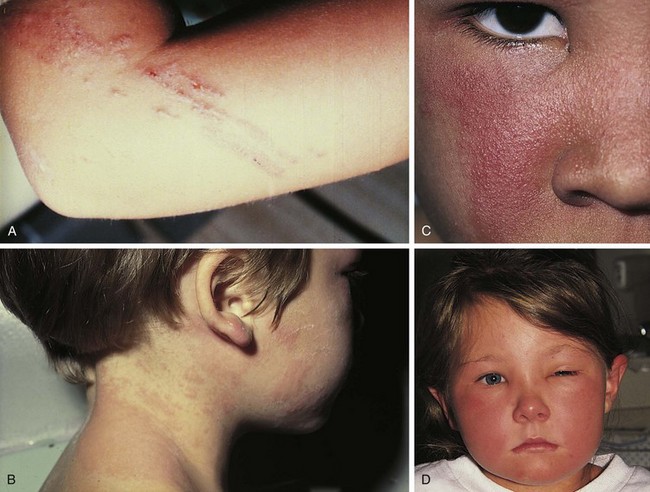
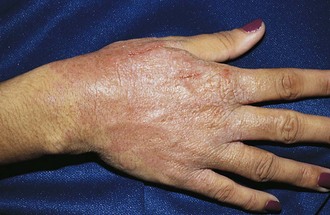
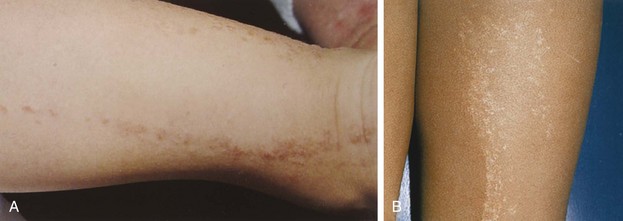
Psoriasis is a common disorder characterized by red, well-demarcated plaques covered with dry, thick, silvery scales. These tend to be located on the extensor surfaces of the extremities, the scalp, and the buttocks. In some patients, the distribution consists of large lesions over the pressure points of the knees and elbows (Fig. 8-3, A–D). Thickening and fissuring of the skin of the palms may also be seen (Fig. 8-3, E). In some children, numerous droplike (guttate) lesions are found scattered over the body (Fig. 8-4), often after a bout of group A β-hemolytic streptococcal infection (which may have been recognized or have been subclinical). In infants, psoriasis may present as a persistent diaper dermatitis (see Fig. 8-45). Lesions of psoriasis are often induced at sites of local injury such as scratches, surgical scars, or sunburn, a response termed the Koebner phenomenon (Fig. 8-5). Nail changes include reddish-brown psoriatic plaques in the nail bed (oil drop changes), surface pitting, and distal hyperkeratosis (see Fig. 8-148).
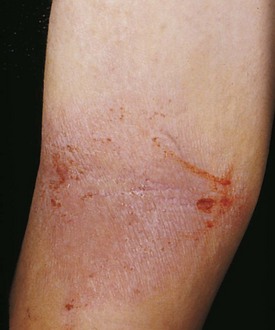
The factors that initiate the rapid turnover in epidermal cells that produce the psoriatic plaques are unknown, although an inherited predisposition is suspected and upper respiratory tract and streptococcal infections may precipitate lesions in children, especially in cases of guttate psoriasis. Although the increased epidermal growth causes a thickening of the skin in the psoriatic plaque, there are also areas between the epidermal ridges where the skin is thin and the scale is close to the subepidermal vessels. Thus when the scale is removed, small bleeding points are often seen. This is called the Auspitz sign, and it is the hallmark of psoriasis (Fig. 8-6).
The Ichthyoses
Ichthyosis Vulgaris
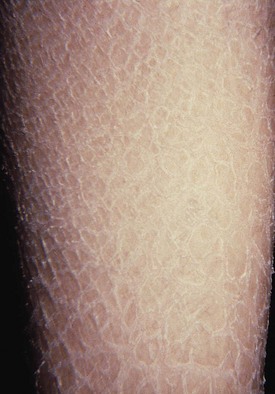
Ichthyosis vulgaris occurs secondary to a mutation in filaggrin and is transmitted as an autosomal dominant trait and affects about 0.5% of the population. Although the rash is not present at birth, by 3 months of age thick, fishlike scales may be apparent on the shins and extensor surfaces of the arms (Fig. 8-7). On occasion, scales become more generalized, involving the trunk, but the flexures are usually spared. Lesions tend to flare during the winter (because of the drying effect of central heating) and improve during the summer, particularly with increasing age. Biopsy of involved skin shows retention hyperkeratosis and a thinned granular layer in the epidermis. Liberal use of topical emollients usually keeps pruritus and scaling under control.
X-linked Ichthyosis
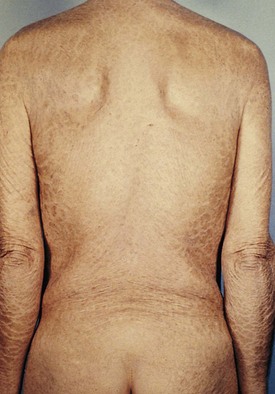
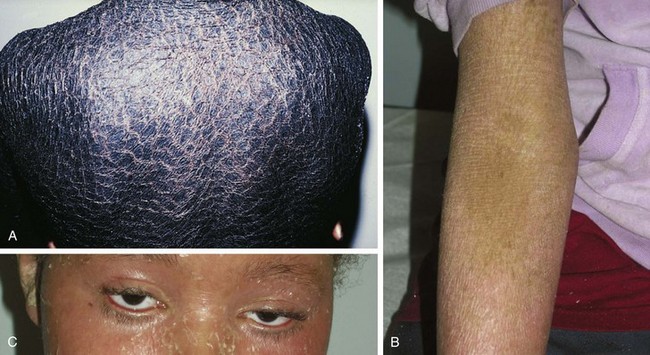
X-linked ichthyosis occurs in 1 in 6000 males as a result of a mutation in the enzyme steroid sulfatase, although findings are occasionally present in hemizygous female carriers. Affected newborns usually present between 3 and 12 months of age with generalized “dirty” brown scales, particularly on the lateral neck, abdomen, back, and anterior legs and feet (Fig. 8-8). The central face and flexures are spared. Skin biopsy demonstrates an increased granular layer and stratum corneum, and biochemical studies demonstrate decreased or absent steroid sulfatase in the serum and skin. Children with X-linked ichthyosis are at increased risk of undescended testes as well (even independent of this risk) as testicular cancer. Decreased or absent steroid sulfatase activity during fetal life may result in decreased placental estrogen and a delay in onset of labor.
Lamellar Ichthyosis
Lamellar ichthyosis is a rare autosomal dominant disorder occurring in fewer than 1 in 250,000 births as a result of a mutation in keratinocyte transglutaminase-1. Infants are usually born with a collodion membrane (see Fig. 8-93). During the first month of life, thick, brownish gray, sheetlike scales with raised edges appear. Scaling is prominent over the face, trunk, and extremities (Fig. 8-9). In contrast to ichthyosis vulgaris, the flexural areas are involved (Fig. 8-9, B). Eversion and fissuring of the eyelid margins (ectropion) and lips (eclabium) are common complications (Fig. 8-9, C). The palms and soles show thick keratoderma with fissuring. Some improvement of the scaling occurs with age, and topical keratolytics such as lactic acid and salicylic acid may provide some benefit. Severe cases may respond to the administration of systemic retinoids such as acitretinoin or isotretinoin.
Epidermolytic Hyperkeratosis
Epidermolytic hyperkeratosis is a rare autosomal dominant form of ichthyosis characterized by the development of generalized, thick, warty scales and intermittent blistering with severe involvement of the flexures (Fig. 8-10). In newborns, blisters and erosions may be widespread and must be differentiated from epidermolysis bullosa or herpes simplex. Histologically, massive hyperkeratosis is associated with ballooning of squamous cells and formation of microvesicles. Epidermal turnover is also markedly increased. The mainstay of treatment includes use of keratolytics, lubricants, and antibiotics for secondary infection, which is common and usually caused by Staphylococcus aureus. Oral retinoids may also significantly decrease scaling.
The Dermatitides
Atopic Dermatitis (Eczema)
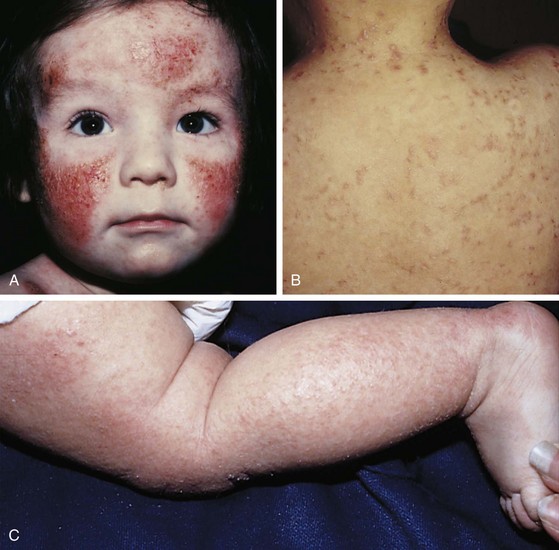
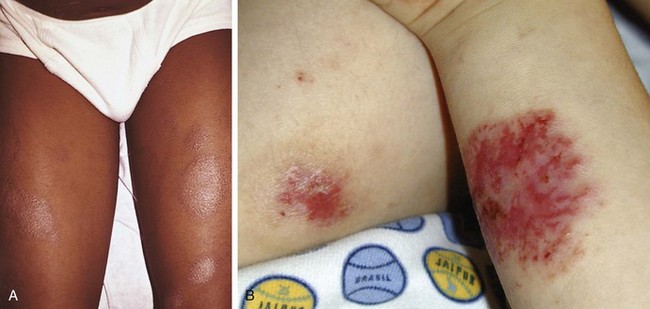
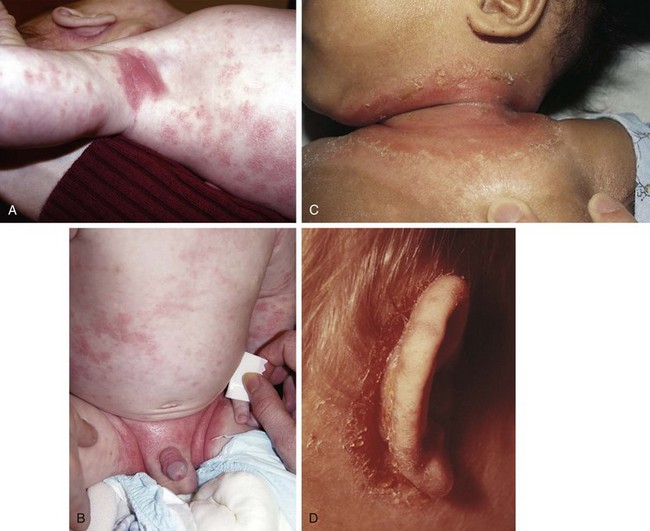
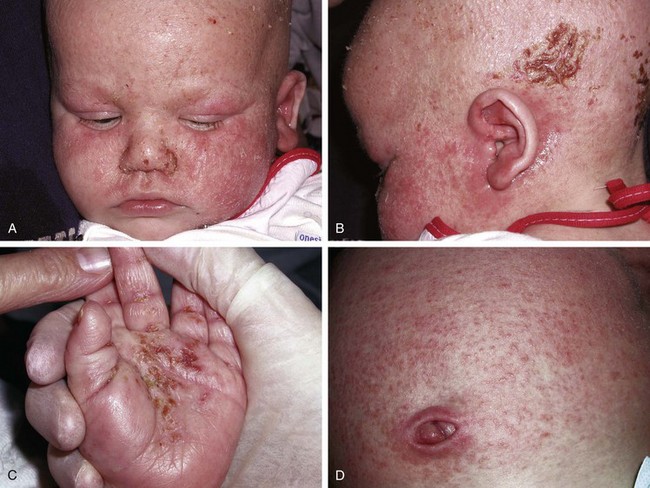
The infantile phase of atopic dermatitis begins between birth and 6 months of age and lasts about 2 or 3 years. Characteristically, the rash is manifest by red, itchy papules and plaques that ooze and crust. Lesions are distributed over the cheeks, forehead, scalp, trunk, and extensor surfaces of the extremities, and patches are often symmetrical (Fig. 8-11).
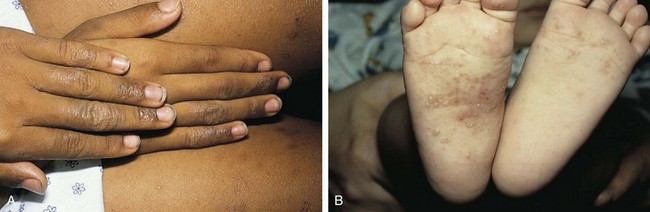
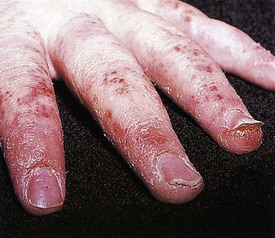
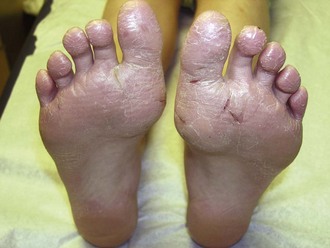
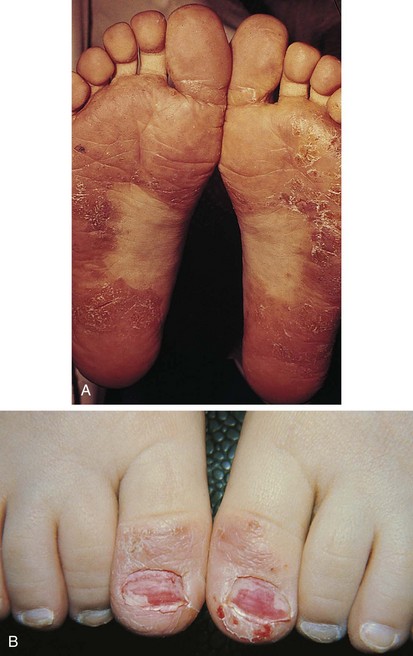
The childhood phase of atopic dermatitis occurs between ages 4 and 10 years. The dermatitis is typically dry, papular, and intensely pruritic. Circumscribed scaly patches are distributed on the wrists, ankles, and antecubital and popliteal fossae (Fig. 8-12); these patches frequently become secondarily infected, both as a result of the nonintact skin barrier as well as secondary to discrete immunologic differences in children with atopic dermatitis, namely a deficiency in two innate antiviral and antibacterial arms of the skin’s immune system: β-defensins and cathelicidins. Cracking, dryness, and scaling of the palmar and plantar surfaces of the hands and feet are also common (Fig. 8-13). Remission may occur at any time, or the disorder may evolve into a more chronic type of adult dermatitis. Of children with atopic dermatitis, 75% improve between the ages of 10 and 14; the remaining children may go on to develop chronic dermatitis.
The adult phase of atopic dermatitis begins around age 12 and continues indefinitely. Major areas of involvement include the flexural areas of the arms, neck, and legs (Fig. 8-14). Eruptions are sometimes seen on the dorsal surfaces of the hands and feet and between the fingers and toes. Lichenification may be marked (Fig. 8-15).
Other associated findings include xerosis (dryness); ichthyosis vulgaris (see Fig. 8-7); keratosis pilaris, keratin plugging of hair follicles and formation of perifollicular scales over the extensor surfaces of the extremities and sometimes the trunk and abdomen (Fig. 8-16; and see also Fig. 8-25, D); hyperlinearity of the palms; Dennie-Morgan lines (double skin creases under the lower eyelid [see Chapter 4]); and hyperpigmentation and hypopigmentation, which may be marked and at times may be the predominant findings. As a result of their altered immunity in the skin, more than 90% of patients with atopic dermatitis are colonized with Staphylococcus aureus at the time of a flare; for the same cellular reason these patients are also unusually susceptible to cutaneous viral infections including warts, herpes simplex, and molluscum contagiosum. Patients with eczema should be warned to avoid people with cold sores because they are at great risk for developing generalized eczema herpeticum (see Fig. 12-13). Parents of children with eczema who themselves have recurrent herpes simplex lesions should be taught hygienic techniques that reduce the risk of transmitting the virus, even when asymptomatic, as shedding can still occur to their children.
(From Cohen BA, Lehman CU, editors: DermAtlas, Baltimore, 2000-2011, Johns Hopkins University. http://dermatlas.med.jhmi.edu/derm.)
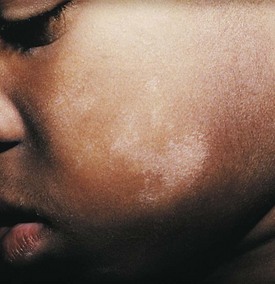
In the rash of pityriasis alba, which is common in patients with atopic dermatitis, inflammatory changes are minimal. Poorly defined, hypopigmented, round or oval scaly patches measuring 2 to 4 cm in diameter are noted most commonly on the face and extremities (Fig. 8-17), although they may involve the trunk as well. These patches fail to enhance with Wood’s lamp examination, which distinguishes them from vitiligo. Lesions are more prominent in children with dark skin and are more noticeable during spring and summer because they do not tan like surrounding skin. Surface scaling is more evident when the skin is dry (especially in winter). The etiology is unknown. Because the disorder is usually asymptomatic and spontaneously resolves in several months to a few years, treatment is usually unnecessary, although moisturizers may help reduce surface scaling.
The differential diagnosis of atopic dermatitis includes seborrheic dermatitis, contact dermatitis, pityriasis rosea, psoriasis, fungal infections, Langerhans cell histiocytosis, and acrodermatitis enteropathica. It can be distinguished from seborrheic dermatitis on the basis of the distribution of lesions and associated pruritus; atopic dermatitis spares moist, intertriginous areas such as the axillae and perineum, where seborrheic dermatitis is prominent. Exposure history and distribution help differentiate it from contact dermatitis, as does the discreteness of lesions and their distribution in pityriasis rosea. The thick, silvery scale and Koebner phenomenon help distinguish psoriasis, and central clearing with an active border of red papules, vesicles, and/or pustules helps differentiate tinea corporis. The rash of histiocytosis is crusted, atrophic, and may be more generalized. It is associated with petechiae and is often accompanied by chronically draining ears, hepatosplenomegaly, and lymphadenopathy (see Fig. 8-85 and Chapter 11). The acral and periorificial distribution of lesions and gastrointestinal symptoms help in distinguishing eczema from acrodermatitis enteropathica.
All children with atopic dermatitis should be monitored closely for secondary bacterial infection, which must be treated promptly with topical or systemic antibiotics to prevent progression to cellulitis. The use of a hyperdiluted bleach (1 to 2 ounces in a 30-gallon tub of water) bath several times per week is a cost-effective way of preventing infection in patients with atopic dermatitis. Herpes simplex can also occur as a secondary infection over atopic dermatitis and can rapidly disseminate in patients with active atopic dermatitis, resulting in the severe disorder known as eczema herpeticum (see Fig. 12-13). Hence, patients’ families should be warned to have their children avoid contact with people with cold sores, and if parents have recurrent herpes labialis, they should be instructed in strict hand-washing precautions. Further, patients should be treated with antiviral agents at the first sign of infection with herpes simplex.
Dyshidrotic Eczema
Dyshidrosis is a severely pruritic, chronic, recurrent, vesicular eruption affecting the palms, soles, and lateral aspects of the fingers and toes. Characteristically, the vesicles are symmetrical, multilocular, and 1 to 3 mm in diameter. These lesions rupture, leaving scales and crust on an erythematous base (Fig. 8-18). Pathologically, this eruption demonstrates spongiotic vesicles and normal eccrine sweat glands. The cause is unknown; however, frequent exposure to water, wet or sweat-soaked shoes, or chemicals (on the hands) may trigger or exacerbate the condition. Hyperhidrosis, or excessive sweating of the palms and soles, may also play a role. Treatment is similar to that for acute atopic dermatitis.
Nummular Eczema
Nummular eczema is an acute papulovesicular eruption named for its coin-shaped configuration and probably also represents another clinical pattern found in atopic individuals. Lesions are intensely pruritic, well-circumscribed, round to oval, red, scaly patches studded with 1- to 3-mm vesicles (Fig. 8-19, A). They are usually located on the extensor thighs or abdomen of children who also may have atopic dermatitis. Vigorous scratching causes excoriation and crusting (Fig. 8-19, B). Lack of central clearing helps distinguish these lesions from tinea corporis (see Fig. 8-35). Although the rash is often resistant to therapy, it may respond to the treatment for acute dermatitis outlined previously.
Juvenile Plantar–Palmar Dermatosis
Juvenile plantar–palmar dermatosis (“sweaty sock syndrome”) is common in toddlers and school-age children. Chronic, red scaly patches with cracking and fissuring typically begin on the anterior plantar surfaces of the feet and big toes (Fig. 8-20). The palms may be involved as well, although less severely. Although the cause is unknown, the condition is triggered by excessive sweating and/or repeated wetting of the skin inside the child’s shoes (especially those made of synthetic materials that do not breathe), followed by drying of the skin at night. Some children experience flares in the summer, whereas in others the flares occur in winter. Consequently, the mainstay of treatment consists of emollients for prevention and occasional application of topical steroids when intense inflammation is present during flares.
Lip-licking and Thumb-sucking Eczema
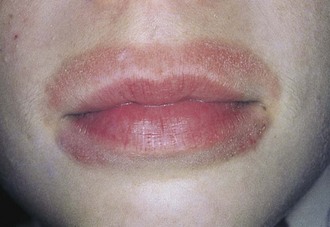
The repeated wetting and drying from persistent lip licking (especially in winter) or thumb sucking can produce eczematoid changes of the perioral skin (Fig. 8-21) or the skin of the involved thumb (Fig. 8-22). Lip-licking eczema can be the result of a habit or can be a manifestation of anxiety, and sources of stress should be explored on history taking. Once the process begins, it can become a vicious cycle as the child licks with increasing frequency to moisten the dry skin.
Seborrhea
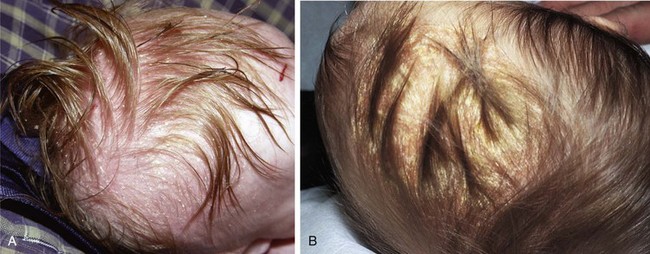
Seborrheic dermatitis is characterized by a red scaling eruption that occurs predominantly on hair-bearing and intertriginous areas such as the scalp; eyebrows; eyelashes; perinasal, presternal, and postauricular areas and the neck; axillae; and groin. Lesions often involve the intertriginous areas of the arms, legs, neck, and trunk, and occasionally become generalized (Fig. 8-23, A–D). In affected infants, scalp lesions consist of a thick, tenacious, scaly dermatitis that is often salmon colored and is commonly known as cradle cap (Fig. 8-24, A and B). In adolescents the dermatitis may manifest as dandruff or flaking of the eyebrows, postauricular areas, or flexural areas.
The dermatitis of seborrhea is usually nonpruritic and mild in nature. Most cases respond to topical steroids, and many clear spontaneously, although residual postinflammatory hypopigmentation may persist for weeks or months thereafter (see Fig. 8-125). Some practitioners find that use of a topical antifungal cream or wash, as well as a low-potency topical steroid, hastens resolution. Antiseborrheic shampoos may also be helpful for patients with scalp involvement. In infants and young children, atopic dermatitis can have a greasy, scaly appearance and may be confused with seborrhea. However, infantile atopic dermatitis produces intense pruritus and invariably spares moist sites such as the diaper area and axillae. The two are often present simultaneously in the same patient. The differential diagnosis of seborrhea includes Langerhans cell histiocytosis (in which the rash is generalized, in part petechial, and usually associated with chronic draining ears and hepatosplenomegaly) and tinea corporis (in which lesions usually are more circumscribed, with an active border and central clearing). Scalp lesions may be difficult to differentiate from psoriasis.
Hyper-IgE (Job) Syndrome
Dermatologic features include a pruritic dermatitic rash that shares features with both atopic dermatitis and seborrhea, and which tends to develop shortly after birth (earlier than seborrhea and atopic dermatitis). It rapidly becomes superinfected with S. aureus, which results in the formation of weeping, crusting, and folliculitic lesions, as well as cutaneous abscesses (Fig. 8-25, A–D). In contrast to furuncles in patients with a normal immune response, the abscesses in children with Job syndrome cause little pain and show few signs of inflammation. Development of mucocutaneous candidiasis is also common. Other clinical manifestations include recurrent/chronic infections such as bronchitis, pneumonia (with pneumatoceles), sinusitis, otitis, gingivitis, dental abscesses, septic arthritis, and osteomyelitis. Decreased bone density is the source of multiple fractures, which cause remarkably little pain. With age and growth, facial features tend to coarsen (see Fig. 4-54, E) and scoliosis is common.
Treatment is aimed at controlling infections and ameliorating symptoms. The major differential diagnostic consideration is Langerhans histiocytosis (see Fig. 8-85).
Pityriasis Rosea
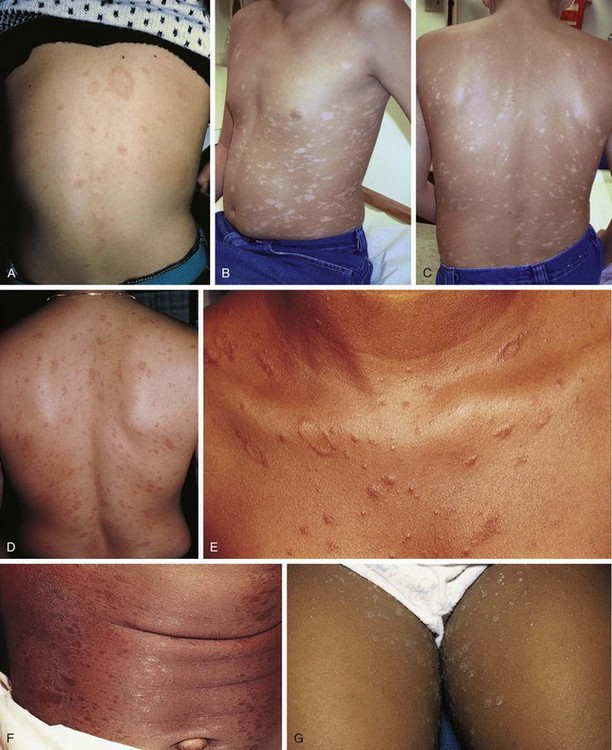
Pityriasis rosea is a benign, self-limited disorder that can occur at any age but is more common in adolescents and young adults. A prodrome of malaise, headache, and mild constitutional symptoms occasionally precedes the rash. The typical eruption begins with the appearance of a “herald patch” (Fig. 8-26, A), which is a large, isolated, oval lesion, usually pink in color and slightly scaly; it may occur anywhere on the body. On occasion, it clears centrally, simulating tinea corporis. From 5 to 10 days later, other smaller lesions appear on the body, frequently concentrated over the trunk but also seen on the proximal extremities, especially the thighs (see Fig. 8-26, B–G). On occasion, lesions predominate on the face, groin, and/or distal extremities including the palms and soles, a phenomenon known as inverse pityriasis, which is more common in African-American patients. Pityriasis lesions begin as small, round papules that enlarge to oval plaques up to 1 to 2 cm in size, with a scaly surface. They are usually somewhat raised but can be macular, and they can be erythematous, hyperpigmented, or hypopigmented. The long axes of the ovals tend to run parallel to the lines of the cleavage of the skin, creating a “Christmas tree” pattern over the thorax (Fig. 8-26, C and D). The rash reaches its peak in several weeks, and then slowly fades over 4 to 8 weeks. The average total duration is 2 to 3 months. Oral erythromycin or doxycycline and small doses of ultraviolet light may hasten the disappearance of the eruption. Although the cause is unknown, the peak incidence in late winter and the low recurrence rate favor an infectious etiology.
Other eruptions that can resemble pityriasis rosea include guttate psoriasis, benign parapsoriasis, viral exanthems, measles-like (morbilliform) drug eruptions, and secondary syphilis (see Fig. 18-36). Secondary syphilis should specifically be considered when a patient presents with a rash resembling pityriasis rosea and the palms and soles are involved. As noted earlier, the appearance of the herald patch may simulate tinea corporis, but a potassium hydroxide (KOH) preparation is negative.
Contact Dermatitis
The initial reaction occurs after a 7- to 14-day period of sensitization in susceptible individuals. Once sensitization has occurred, reexposure to the allergen provokes a more rapid reaction, sometimes within hours. This is a classic example of type IV (delayed) hypersensitivity (see Chapter 4).
Rhus Dermatitis (Poison Ivy)
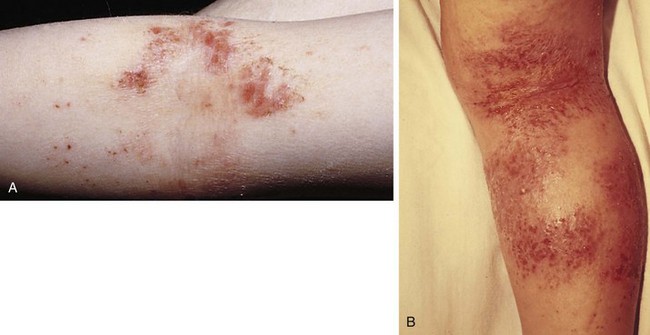
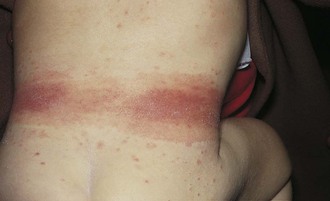
The most common type of allergic contact dermatitis in the United States is poison ivy, or rhus dermatitis. This typically presents as linear streaks of erythematous papules and vesicles (Fig. 8-27, A); however, with heavy exposure, the rash may appear in relatively large patches (Fig. 8-27, B and C). When lesions involve the skin of the face or genitalia, impressive swelling can occur (Fig. 8-27, D).
Direct contact with the sap of poison ivy, poison oak, or poison sumac from leaves, stems, or roots (whether the plant is alive or dead) produces the dermatitis (Fig. 8-28). Contact with clothing that has brushed against the plant, with logs or railroad ties on which the vine has been growing, or with smoke from a fire in which the plant is being burned are other means of exposure. Areas of skin exposed to the highest concentration of plant oil develop changes first. Other sites that received lower doses then vesiculate in succession, giving the illusion of spreading. This is not the case, however, as within about 20 minutes of initial contact, the rhus oil becomes tissue-fixed to the epithelial cells and cannot be spread farther. Thorough washing within minutes of exposure can prevent fixation, and hence, the eruption. On occasion the oil can oxidize, leaving a black discoloration superimposed on the contact dermatitis; this is known as “black dot” or “urushiol” dermatitis.
Other Common Causes of Contact Dermatitis
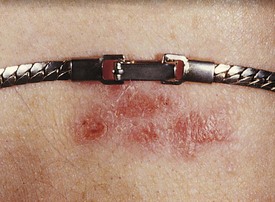
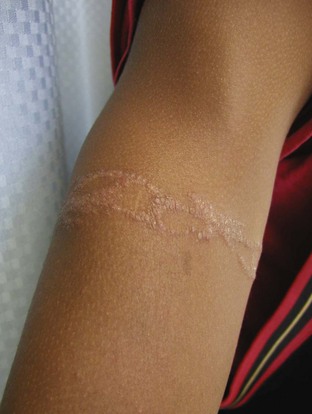
Other common offending agents are nickel (Fig. 8-29), rubber (Fig. 8-30), glues and/or dyes in shoes or diapers (Fig. 8-31), ethylenediamine in topical lotions, neomycin, and topical anesthetics (Fig. 8-32). Paraphenylenediamine dye used in amateur henna tattoos is an increasingly common cause of contact dermatitis among adolescents, and the pattern reflects the image of the original tattoo (Fig. 8-33).
Photocontact Dermatitis and Phototoxic Reactions
Photocontact or photoallergic dermatitis is a true cell-mediated delayed hypersensitivity reaction and necessitates a 7- to 10-day period of sensitization, after which sun exposure may precipitate development of a dermatitis. When caused by systemically administered drugs, it characteristically erupts in a symmetrical distribution on the face, the V of the neck, and the arms and legs distal to the end of shirt sleeves and shorts. Potentially causative agents include the tetracyclines, isotretinoin, sulfonylureas, thiazides, nonsteroidal antiinflammatory drugs, fluoroquinolones, and griseofulvin. Topical photosensitizers (sunscreens with PABA esters or oxybenzone; fragrances in soaps, creams, lotions, or cosmetics; coal tar; furocoumarins; and halogenated salicylanilides in germicidal soaps) produce localized patches of dermatitis when used on sun-exposed sites (Fig. 8-34).
Phototoxins, when applied to the skin, are the source of a nonimmunologic exaggerated sunburn reaction in which the initial erythema progresses to hyperpigmentation. Of these, phytophotodermatitis is the most common. In this form, plant-derived photosensitizers, psoralens, found in the juice of lemons, limes, figs, dill, parsley, parsnips, carrots, and celery, are responsible for the reaction. Typically a child is touched by a parent who has been cutting the fruit, herbs, or vegetables, thereby getting the juice on the child’s skin. Subsequent exposure to sunlight then results in the appearance of erythematous macules, with or without accompanying bullae, which then go on to become hyperpigmented. These patches often have bizarre or hand/finger-shaped patterns that can mimic child abuse (see Fig. 6-66).
Fungal Infections
Tinea Corporis
Tinea corporis is a superficial fungal infection of the nonhairy or glabrous skin. It has been labeled “ringworm” because of its characteristic configuration consisting of pruritic, annular lesions with central clearing and an active border made up of microvesicles that rupture and then scale (Fig. 8-35, A–C). Lesions, which may be single or multiple, typically begin as pruritic red papules or pustules that rupture and evolve to form papulosquamous lesions, which are also pruritic. These then spread out from the periphery as new vesicles form at their outer margins, and at the same time begin to clear centrally (see Fig. 8-35, D and E). Over a period of several weeks, the patches may expand up to 5 cm in diameter. Tinea corporis can be found in any age group and is usually acquired through direct human contact (Trichophyton tonsurans) or from an infected pet, such as a kitten.
Clinically, tinea may be differentiated from atopic dermatitis by the propensity for autoinoculation from the primary patch to other sites on the patient’s skin, by the spread to close contacts, and by the central clearing noted in many lesions. Moreover, the rash of atopic dermatitis tends to be symmetrical, chronic, and recurrent in a flexural distribution. Unlike tinea, patches of nummular eczema are self-limited and do not clear centrally. The herald patch of pityriasis rosea is often mistaken for tinea. However, it is KOH negative, and the subsequent development of the generalized rash with its characteristic truncal distribution is distinctive (see Fig. 8-26). The clinical pattern, findings, and chronic nature of psoriasis and seborrhea help differentiate them from tinea. Although granuloma annulare produces a characteristic ringed eruption, on palpation the lesions are firm and usually asymptomatic. They tend not to show epidermal changes other than occasional slight scaling (see Fig. 8-86).
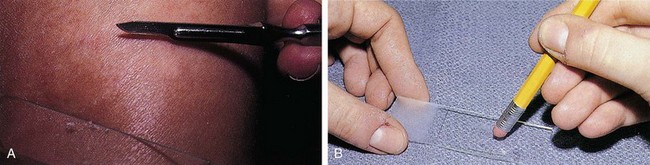
The diagnosis of tinea corporis is confirmed by KOH examination of skin scrapings. The first step is to obtain material by scraping the loose scales at the margin of a lesion (Fig. 8-36, A). These should be mounted onto the center of a glass slide, with one or two drops of 20% KOH solution added. Next, a glass coverslip is applied and gently pressed down with the eraser end of a pencil to crush the scales (Fig. 8-36, B). The clinician then heats the slide, taking care not to boil the KOH solution, and again the coverslip is pressed down. When viewing the slide under the microscope, the clinician sets the condenser and light source at low levels to maximize contrast, with the objective at ×10. On focusing up and down, true hyphae are seen as long, branching, often septate rods of uniform width that cross the borders of epidermal cells (Fig. 8-37). Cotton fibers, cell borders, or other artifacts may be falsely interpreted as positive findings.
Tinea Pedis
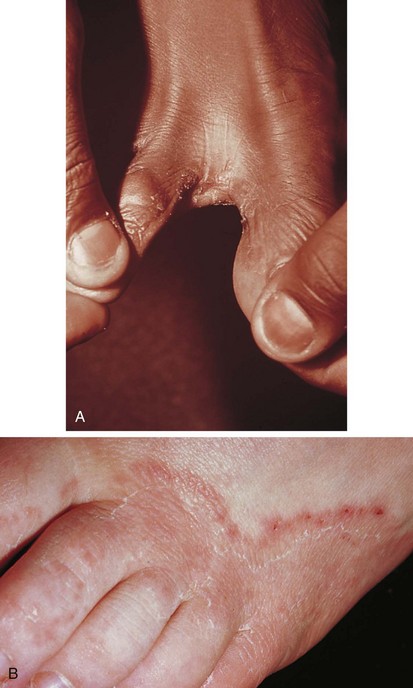
In some cases, scaling and fissuring predominate; in others, vesiculopustular lesions and maceration are found, especially in the web spaces between the third, fourth, and fifth toes. The infection begins between and along the sides of the toes, where it may remain (Fig. 8-38, A). However, lesions can extend over the dorsum of the foot (see Fig. 8-38, B) and may involve the plantar surface as well, particularly the instep and ball of the foot. Patients complain of a combination of burning and itching, which is frequently intense.
This diagnosis often can be made on clinical grounds and is confirmed by KOH preparation of skin scrapings. The mainstays of treatment are topical antifungal creams or powders, as well as measures designed to reduce foot moisture. The latter include careful drying of the feet after bathing, wearing cotton rather than synthetic socks, and wearing shoes that do not promote sweating or, better still, sandals. In patients with severe inflammatory lesions, oral antifungal agents may be required. Onychomycosis (see Fig. 8-144) responds well only to oral agents and requires treatment for 4 months, occasionally longer (see Disorders Affecting the Nails, later). Secondary bacterial infection (particularly with gram-negative organisms) may be a problem.
Tinea pedis is distinguished from contact dermatitis of the feet by virtue of the fact that the latter spares the interdigital web spaces (see Fig. 8-31). Dyshidrosis can have a similar distribution, but the KOH preparation is negative (see Fig. 8-18).
Tinea Versicolor
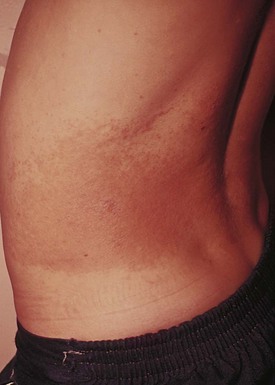
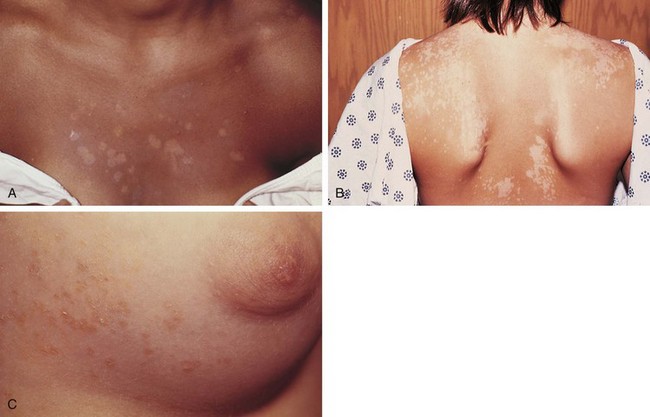
Tinea versicolor is a common dermatosis characterized by multiple small, oval, scaly patches measuring 1 to 3 cm in diameter, usually located in a guttate or raindrop pattern on the upper chest, back, and proximal portions of the upper extremities of adolescents and young adults (Fig. 8-39, A and B). However, all ages may be affected, including infants. Facial involvement occurs occasionally. The eruption is caused by a dimorphous form of Pityrosporum. Warm, moist climates, pregnancy, immunodeficiency, and genetic factors predispose people to the development of infection.
The rash is usually asymptomatic, although some patients complain of mild pruritus. Typically, patients go to the physician because they are bothered by the cosmetic appearance of the lesions. Lesions may be light tan, reddish, or lightly discolored, giving rise to the term versicolor. They are darker than surrounding skin in non–sun-exposed areas (Fig. 8-39, C) and lighter in areas that have tanned on exposure to sunlight (see Fig. 8-39, A and B).
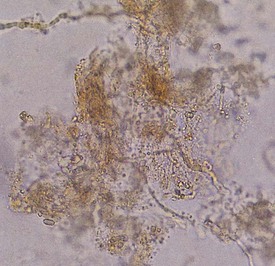
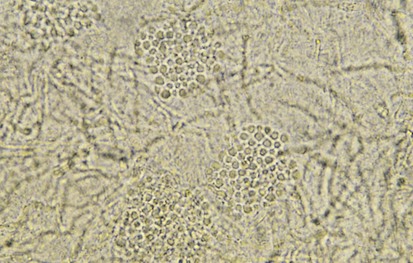
The diagnosis of tinea versicolor can generally be made on the basis of the clinical appearance of lesions and their distribution. It can be confirmed by examining the lesions under a Wood’s lamp, which reveals a characteristic tan to salmon-pink glow but no enhancement of the discoloration as in vitiligo. Although pathogenesis of the color change under a Wood’s lamp is not fully understood, the fungus is known to produce a substance that interferes with tyrosinase activity and subsequent melanin synthesis. A KOH preparation of the surface scale demonstrates short hyphal and yeast forms that resemble spaghetti and meatballs (Fig. 8-40).
Diaper Dermatitis
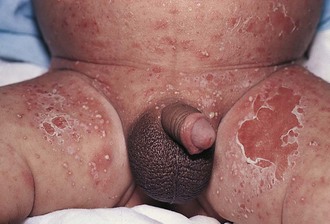
Irritant Diaper Dermatitis
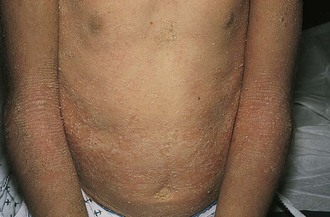
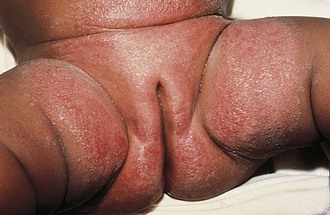
The diaper area is a prime target for irritant dermatitis because it is bathed in urine and stool and occluded by plastic diaper covers. Failure to change diapers frequently is a major predisposing factor because it provides time for fecal bacteria to form ammonia by splitting the urea in urine. Harsh soaps, irritant chemicals, and detergents can contribute to the process. Moderate to severe diarrhea is another predisposing condition. The erythema; scaling; and, at times, maceration characteristic of irritant diaper dermatitis are usually confined to the convex surfaces of the perineum, lower abdomen, buttocks, and proximal thighs, sparing intertriginous areas (Fig. 8-41). When neglected, this may progress, with further skin breakdown and ulceration. Frequent diaper changes; gentle, thorough cleansing of the area; and application of lubricants and barrier pastes usually result in clearing of the dermatitis. A short course of low-potency steroids may hasten resolution, but this must be discontinued after a week or so and then followed by prevention with thick, unfragranced zinc oxide pastes as a barrier.
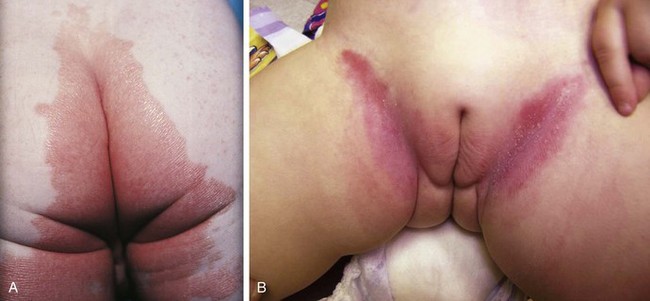
Candidal Diaper Dermatitis
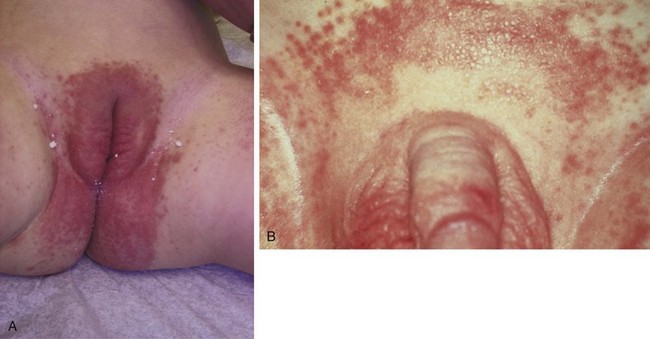
Candidal diaper dermatitis appears as a bright red eruption, with sharp borders and pinpoint satellite papules and pustules (Fig. 8-42, A and B). Examination of pustule contents by KOH preparation reveals the typical budding yeasts and pseudohyphae of Candida organisms (Fig. 8-43). Candidal diaper dermatitis is occasionally associated with oral thrush, and it is a common sequela of oral or parenteral antibiotic therapy. One should suspect a secondary invasion by Candida albicans whenever intertriginous areas are involved or when a diaper rash fails to respond to symptomatic treatment. Most cases respond well to topical antifungal therapy, but the occasional resistant case may require a brief course of oral medication.
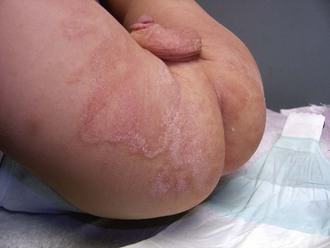
Staphylococcal Diaper Dermatitis
Irritant diaper dermatitis is frequently complicated by secondary staphylococcal infection, or pustules may appear as primary lesions, especially in the first few weeks of life. The presence of thin-walled pustules on an erythematous base (larger than those seen with candidiasis) alert the clinician to the diagnosis. Typically, these rupture rapidly and dry, producing a collarette of scaling around the denuded red base (Fig. 8-44). A Gram stain of pustule contents demonstrates neutrophils and clusters of gram-positive cocci. Bacterial cultures are confirmatory but are rarely necessary. Early diagnosis and treatment with oral and topical antibiotics result in rapid resolution.
Seborrheic Diaper Dermatitis
Seborrheic diaper dermatitis is characterized by salmon-colored lesions with a yellowish scale. The rash is particularly prominent in the intertriginous areas (see Fig. 8-23, B). Although concurrent infection with Candida or Pityrosporum is likely, satellite lesions are usually not seen. Typically, seborrheic dermatitis of the scalp, face, and postauricular areas is seen in association with this form of diaper dermatitis.
Psoriatic Diaper Dermatitis
Psoriasis occasionally begins as an erythematous, scaling eruption in the diaper area (Fig. 8-45, A and B), which is clinically indistinguishable from seborrheic diaper dermatitis. Perhaps because the diaper area tends to be moist, the thick silvery scale typical of psoriatic lesions at other sites is not seen. Although lesions may develop subsequently on the trunk and extremities, the rash may persist for months in the diaper area alone. Failure of a seborrheic-like diaper rash to respond to empiric therapy over several weeks or months should raise psoriasis as a diagnostic possibility. Skin biopsy is the only way to confirm the diagnosis.
Tinea Diaper Dermatitis
Although less common than the other dermatitides in the differential diagnosis of diaper dermatitis, tinea must be kept in mind when examining a scaly perineal rash. Its characteristic features are those of a recalcitrant scaly eruption with an elevated or “active” scaly border (Fig. 8-46), from which scales can be scraped and tinea demonstrated on KOH preparation. It responds well to topical antifungals alone and should not be treated with topical steroids.
Lichenoid Eruptions
Lichen Planus
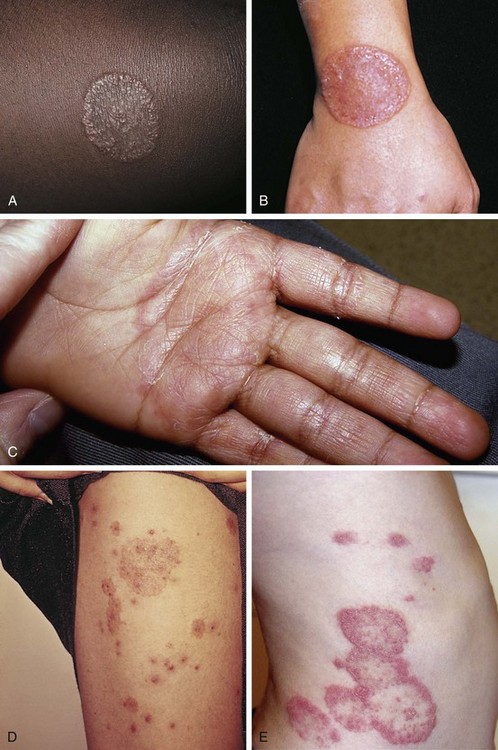
Lichen planus is the prototypic lichenoid inflammatory eruption in that it displays flat-topped pruritic polygonal violaceous papules and plaques, which have a fine lacy pattern (Wickham striae) over their outer surfaces. They are often noted first over the dorsal surfaces of the extremities and can display koebnerization at sites of prior trauma (Fig. 8-47). The disorder may involve the nails (leading to dystrophy) as well as the oral mucosa, where lesions appear as lacy white plaques. Topical steroids are the mainstay of initial treatment. After resolution of primary lesions, a prolonged period of postinflammatory hyperpigmentation can be expected (see Fig. 8-124).
Lichen Striatus
Lichen striatus is one of the more common and distinctive lichenoid papulosquamous eruptions. Lesions consisting of flat-topped papules appear fairly abruptly, usually in a linear or sometimes swirled distribution along the lines of Blaschko. Although they may involve any portion of the skin, they are more typically located on the extremities, neck, or upper back. They may be slightly erythematous or hypopigmented, and their surfaces are covered with fine scale (Fig. 8-48, A and B). When lesions form on a digit, lichen striatus can lead to temporary linear nail dystrophy. The cause of this otherwise asymptomatic disorder, which has its peak incidence in school-age children, is unknown. Spontaneous resolution within 1 to 3 years is the norm.
Vesiculopustular Disorders
Viral Infections
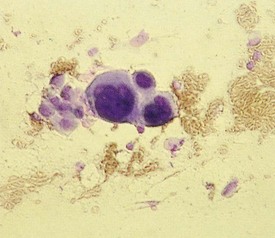
Viral infections including herpes simplex and varicella-zoster produce characteristic vesiculopustular exanthems, which are discussed in Chapter 12. However, the technique of confirming the suspicion of a herpetic lesion by preparing a Tzanck test is discussed here.
The Tzanck smear is obtained by removing the roof of a vesicle with a scalpel or scissors and scraping the cells at its base. This is then spread onto a glass slide with the scalpel blade, air dried, and stained with Giemsa or Wright stain. The diagnostic finding in viral blisters is the multinucleated giant cell (Fig. 8-49). This is a syncytium of epidermal cells with multiple, overlapping nuclei; hence it is much larger than other inflammatory cells. Unfortunately, a positive Tzanck test cannot be used to differentiate one blistering viral exanthem from another, and a viral culture, or the more rapid direct fluorescent antibody test, should be done when the clinical situation mandates precise identification.
Bacterial Infections
Several common cutaneous bacterial infections present with vesiculopustular reactions as well. In impetigo, the eruption tends to be discrete and localized, whereas in staphylococcal scalded skin syndrome (SSSS) it tends to be associated with a diffuse erythroderma. A Gram stain of material aspirated from bullae or removed from the base of an impetiginous lesion is positive for organisms. However, in patients with SSSS, the organism must be sought from noncutaneous colonized locations (nasopharynx, conjunctivae, sinuses) because the diffuse cutaneous blistering is due to elaboration of the toxin epidermolysin by the infecting organism and not to the organism’s direct action within individual lesions (see Chapter 12 and Fig. 12-18).
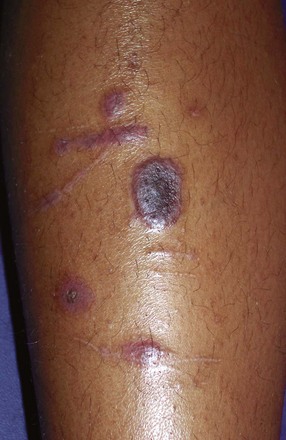
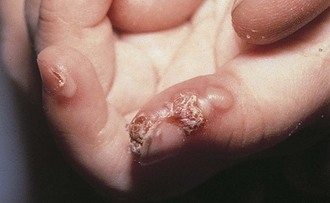
Blistering Distal Dactylitis
Blistering distal dactylitis is a superficial bacterial infection involving the tips of the pads of fingers or toes. Lesions consist of tense blisters 0.5 to 1 cm in diameter that are filled with thin purulent fluid and surrounded by a narrow erythematous rim. With evolution, they may coalesce and extend proximally along the lateral aspect of the nail fold. On rupture, a thick crust forms (Fig. 8-50). Group A β-streptococci are the usual causative organisms and are detected by Gram stain and culture of vesicular fluid. On occasion, group B streptococci and S. aureus are isolated. The disorder can be distinguished from a paronychia by the distal location of initial lesions; from a herpetic whitlow by the larger size of the initial vesicles and Gram stain of the purulent fluid; and from burns by the purulence of the vesicular fluid and Gram stain revealing white blood cells and bacteria.
Erythema Multiforme
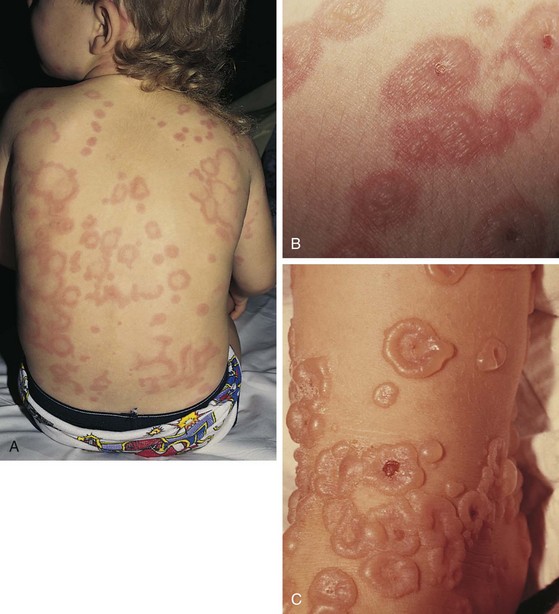
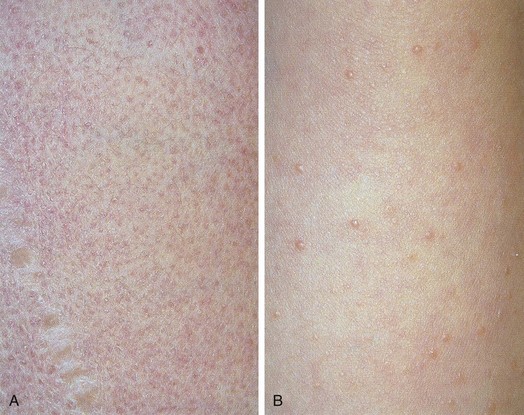
The classic eruption of EM is symmetrical and may occur on any part of the body. However, the dorsum of the hands and feet and the extensor surfaces of the arms and legs are affected most commonly. Involvement of the palms and soles also is typical. The initial lesions are dusky red macules or erythematous wheals that evolve into iris- or target-shaped lesions, the hallmark of EM (Fig. 8-51, A). In many instances the initial crop of lesions simulates diffuse urticaria, although EM lesions are typically much less pruritic, if at all, and ultimately they become painful and persistent, in contrast to true urticarial lesions, which remain pruritic and tend to migrate over a 24-hour period. The target configuration is due to formation of a central depression that may be blue, violaceous, or white, whereas the elevated periphery tends to remain erythematous. In some cases, vesicles or bullae develop centrally, and in others the peripheral rings may vesiculate or become bullous (Fig. 8-51, B and C). The eruption continues in crops that last from 1 to 3 weeks. In most patients the disease is self-limited, and systemic manifestations are relatively mild, consisting of low-grade fever, malaise, and myalgia. Mucous membranes tend to be spared, although, on occasion, the oral mucosa may be mildly involved. The major differential diagnosis is urticaria, which does not demonstrate three discrete zones of color change to form a target, as do lesions of true erythema multiforme.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree