Cytoreductive Surgery for Ovarian Cancer: Upper Abdominal Procedures
Robert E. Bristow
INTRODUCTION
Approximately 70% of patients with epithelial ovarian cancer will have advanced-stage disease at the time of diagnosis. For this group, survival determinants are multifactorial; however, the strongest clinician-driven predictors of clinical outcome are the administration of platinum-based chemotherapy and the amount of residual tumor following primary surgery, with complete tumor resection being associated with the most favorable outcome. As many as 42% of patients with Stage IIIC ovarian cancer will have bulky (>1 cm) disease in the upper abdomen above the greater omentum. These patients often require a more extensive upper abdominal cytoreductive surgical effort to achieve optimal (≤1 cm) or no gross residual disease prior to initiating chemotherapy. For example, while the omentum represents the location of the largest tumor site in many patients with advanced-stage ovarian cancer, direct invasion of the transverse colon and adjacent structures is not infrequent and dictates a broader scope of surgical resection. Splenectomy may also be required, as tumor may extend from the greater omentum to affect the spleen in 5% to 30% of patients with advanced-stage disease. Similarly, metastatic tumor spread to the diaphragm will be encountered in 18% to 41% of patients with Stage III and IV epithelial ovarian cancer. As a result, a maximal surgical effort is dependent on the surgeon’s capacity to successfully manage disease involving the structures of the upper abdomen.
PREOPERATIVE CONSIDERATIONS
In preparation for surgery for ovarian cancer, all patients should undergo a comprehensive history and physical examination focusing on those areas that may indicate a reduced capacity to tolerate major surgery or place the patient at elevated risk for postoperative complications. Routine laboratory testing should include a complete blood count, serum electrolytes, age-appropriate health-screening studies, a chest radiograph, and electrocardiogram for women aged 50 years and older. Serum tumor markers are not a prerequisite; however, a preoperative serum CA125 level is recommended, not so much for its diagnostic value, but rather to serve as a baseline level in the event that an ovarian cancer diagnosis is confirmed pathologically. Preoperative computed tomography of the abdomen, pelvis, and chest is recommended to evaluate the extent of disease and for surgical planning purposes.
Because ovarian cancer surgery carries the possibility of bowel resection or injury, preoperative mechanical bowel preparation (oral polyethylene glycol solution or sodium phosphate solution with or without bisacodyl) is recommended according to the surgeon’s preference. Prophylactic antibiotics (Cephazolin 1 g, Cefotetan 1 to 2 g, or Clindamycin 800 mg) should be administered 30 minutes prior to incision, and thromboembolic prophylaxis (e.g., pneumatic compression devices and subcutaneous heparin) should be initiated prior to surgery. Reservation of an intensive care unit bed postoperatively is advisable if extensive or
prolonged surgery is anticipated, and type and cross-matched blood should be available. If splenectomy is anticipated, a single one-time polyvalent pneumococcal vaccination (Pneumovax) and vaccines against Haemophilus influenza (Hib) and Neisseria meningitidis (Menomune) should be given 10 to 14 days preoperatively. In the circumstance of an unplanned splenectomy, the vaccines should be given 14 days postoperatively.
prolonged surgery is anticipated, and type and cross-matched blood should be available. If splenectomy is anticipated, a single one-time polyvalent pneumococcal vaccination (Pneumovax) and vaccines against Haemophilus influenza (Hib) and Neisseria meningitidis (Menomune) should be given 10 to 14 days preoperatively. In the circumstance of an unplanned splenectomy, the vaccines should be given 14 days postoperatively.
A self-retaining retractor (e.g., Bookwalter, Codman Division, Johnson and Johnson, Piscataway, NJ) with a fixed arm attaching the retractor ring to the operating table is essential to optimize exposure, maximize patient safety, and reduce surgeon fatigue. At the surgeon’s discretion, additional standard equipment may include an electrosurgical unit (ESU or “Bovie”), vessel-sealing device, argon beam coagulator (ABC), cavitron ultrasonic surgical aspirator (CUSA), and automated stapling devices. Following are brief descriptions of the surgical procedures used (see also videos: Cytoreductive Surgery for Ovarian Cancer— Upper Abdominal Disease: Transverse Colectomy; Splenectomy; Diaphragm).
SURGICAL TECHNIQUE
The patient may be positioned in the dorsal low-lithotomy (perineolithotomy) position using Allen Universal Stirrups (Allen Medical Systems, Cleveland, OH) or supine on the operating table. Mechanical ventilation should be confirmed prior to undertaking upper abdominal cytoreductive procedures. Abdominal entry and exposure are achieved through a midline xiphopubic incision, which can be extended alongside the xyphoid process for additional exposure, with placement of a self-retaining retractor positioned to provide firm, upward traction on the costal margins. A preliminary assessment is taken of the extent of disease, with particular attention to the feasibility of resecting upper abdominal disease. Many surgeons prefer to begin the operation with the removal of the omentum, as it is usually extensively involved with tumor and provides a convenient starting point for surgical extirpation of metastatic disease in the upper abdomen. From an anatomic perspective, contiguous extension to the transverse colon, spleen, or diaphragm may require an en bloc resection of one or a combination of these organs with the omental tumor. Even if the omental disease does not directly involve adjacent structures, its removal will improve exposure and facilitate mobilization of the surrounding anatomy so that subsequent resection of isolated metastases can be accomplished with maximum safety.
Omentectomy with en bloc transverse colectomy
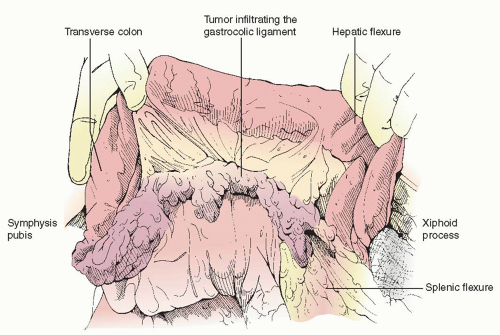
Bulky omental disease extending to or infiltrating  into the transverse colon or pericolonic soft tissue and mesentery necessitates partial or total transverse colectomy en bloc with an omentectomy and resection of gastrocolic ligament, provided this component of the operation will make a significant contribution to the overall surgical result of achieving minimal residual disease. Tumor spread into the lesser sac and involving the transverse colon mesentery is also an indication for transverse colectomy.
into the transverse colon or pericolonic soft tissue and mesentery necessitates partial or total transverse colectomy en bloc with an omentectomy and resection of gastrocolic ligament, provided this component of the operation will make a significant contribution to the overall surgical result of achieving minimal residual disease. Tumor spread into the lesser sac and involving the transverse colon mesentery is also an indication for transverse colectomy.
 into the transverse colon or pericolonic soft tissue and mesentery necessitates partial or total transverse colectomy en bloc with an omentectomy and resection of gastrocolic ligament, provided this component of the operation will make a significant contribution to the overall surgical result of achieving minimal residual disease. Tumor spread into the lesser sac and involving the transverse colon mesentery is also an indication for transverse colectomy.
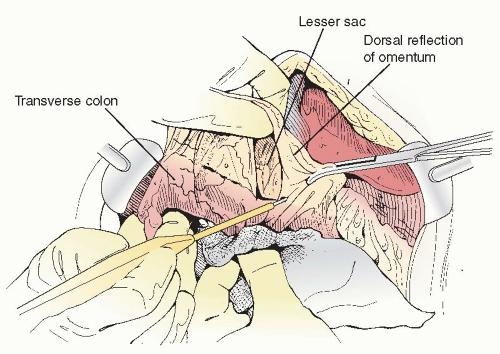
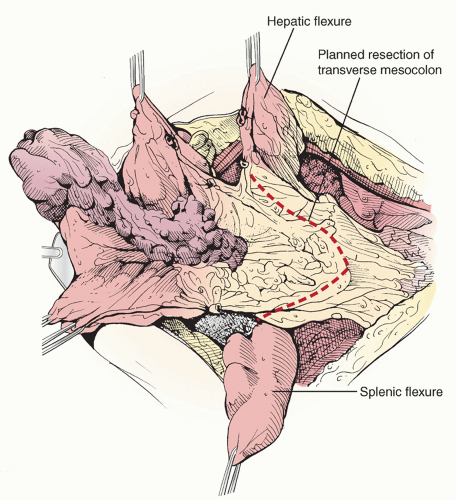
into the transverse colon or pericolonic soft tissue and mesentery necessitates partial or total transverse colectomy en bloc with an omentectomy and resection of gastrocolic ligament, provided this component of the operation will make a significant contribution to the overall surgical result of achieving minimal residual disease. Tumor spread into the lesser sac and involving the transverse colon mesentery is also an indication for transverse colectomy.En bloc omentectomy and transverse colectomy begins by dividing the attachments of the omentum to the lateral aspects of the transverse colon. The right and left gastroepiploic vascular pedicles are clamped, divided, and secured with suture ligatures or taken down with a vessel-sealing device. The dorsal reflection of the omentum onto the transverse colon is incised with the ESU, and the potential space of the lesser sac is developed (Figure 20.1). The transverse colon is then completely mobilized by dividing the gastrocolic ligament from the greater curvature of the stomach and by taking town the hepatocystocolic, phrenicocolic, and splenocolic ligaments to free the hepatic flexure and splenic flexure, respectively (Figure 20.2).
Once the omental attachments have been freed, the en bloc tumor specimen to be resected is clearly delineated and the vascular supply to the remaining proximal and distal ends of the transverse colon inspected. The surgeon must make certain that the marginal artery of Drummond is intact and will provide sufficient blood supply to both ends of the planned anastomosis (transverse colocolostomy). The proximal and distal segments of the involved section of transverse colon are cleared of surrounding fat and divided with the Gastrointestinal anastomosis (GIA) stapling device (Figure 20.3). A wedge-shaped section of transverse colon mesentery is demarcated and incised, the middle colic artery and vein identified, divided, and ligated, and the specimen removed (Figure 20.4). Any associated arterial arcades are similarly individually divided and suture ligated or controlled with electrocautery. If the marginal artery of Drummond is discontinuous at the splenic flexure, the distal transverse colon and proximal descending colon should be included in the scope of resection.
Intestinal continuity is reestablished via either an end-to-end or functional end-to-end stapled or hand-sewn anastomosis. A functional end-to-end stapled colocolostomy using the GIA and Thoraco-abdominal (TA) stapling devices is safe, efficient, and produces a capacious anastomotic lumen (Figure 20.5). In addition to having an adequate blood supply, the avoidance of tension on the staple or suture line is critical to creating a viable anastomosis, and additional mobilization of the hepatic flexure and/or splenic flexure may be required. Finally, the mesenteric defect is closed with interrupted stitches of 2-0 delayed absorbable suture; however, the duodenojejunal junction should be carefully inspected to confirm that re-approximating the colonic mesentery does not produce a functional stricture at this point.
Splenectomy
 The most common location of ovarian cancer involving the spleen is the hilum (65%), followed by the capsule (52%), and parenchyma (16%). The surgical approach to splenectomy is individualized and may proceed anteriorly through the gastrosplenic ligament, posteriorly through the lienorenal ligament, or a combination of the two, depending
The most common location of ovarian cancer involving the spleen is the hilum (65%), followed by the capsule (52%), and parenchyma (16%). The surgical approach to splenectomy is individualized and may proceed anteriorly through the gastrosplenic ligament, posteriorly through the lienorenal ligament, or a combination of the two, depending on the location and extent of tumor growth. If the anterior surface of the spleen and splenic hilum are not obscured by tumor, the anterior approach is the most direct route to control the splenic blood supply. Following mobilization of the omentum, the splenocolic, gastrocolic, and gastrosplenic ligaments are divided (Figure 20.6
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree