Chapter 425 Cyanotic Congenital Heart Disease
Lesions Associated with Increased Pulmonary Blood Flow
425.1 D-Transposition of the Great Arteries
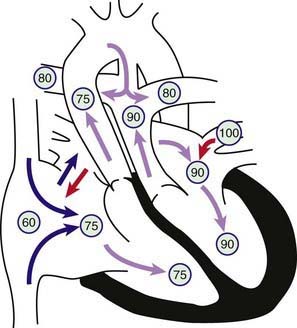
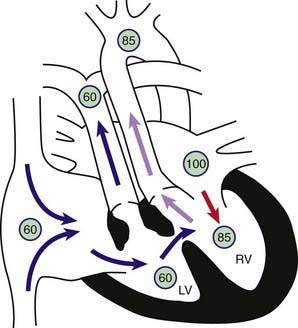
Transposition of the great vessels, a common cyanotic congenital anomaly, accounts for ≈5% of all congenital heart disease. In this anomaly, the systemic veins return normally to the right atrium and the pulmonary veins return to the left atrium. The connections between the atria and ventricles are also normal (atrioventricular concordance). The aorta arises from the right ventricle and the pulmonary artery from the left ventricle (Fig. 425-1). In normally related great vessels, the aorta is posterior and to the right of the pulmonary artery; in d-transposition of the great arteries (d-TGA), the aorta is anterior and to the right of the pulmonary artery (the d indicates a dextropositioned aorta, transposition indicates that it arises from the anterior right ventricle). Desaturated blood returning from the body to the right side of the heart goes inappropriately out the aorta and back to the body again, whereas oxygenated pulmonary venous blood returning to the left side of the heart is returned directly to the lungs. Thus, the systemic and pulmonary circulations exist as two parallel circuits. Survival in the immediate newborn period is provided by the foramen ovale and the ductus arteriosus, which permit some mixture of oxygenated and deoxygenated blood. About 50% of patients with d-TGA also have a ventricular septal defect (VSD), which usually provides for better mixing. The clinical findings and hemodynamics vary in relation to the presence or absence of associated defects (e.g. VSD or pulmonary stenosis). d-TGA is more common in infants of diabetic mothers and in males (3 : 1). d-TGA, especially when accompanied by other cardiac defects such as pulmonic stenosis or right aortic arch, can be associated with deletion of chromosome 22q11 (DiGeorge syndrome [Chapter 418]). Before the modern era of corrective or palliative surgery, mortality was >90% in the 1st yr of life.
425.2 D-Transposition of the Great Arteries with Intact Ventricular Septum
Diagnosis
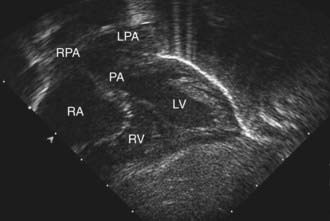
The electrocardiogram is usually normal, showing the expected neonatal right-sided dominant pattern. Roentgenograms of the chest may show mild cardiomegaly, a narrow mediastinum (the classic “egg-shaped heart”), and normal to increased pulmonary blood flow. In the early newborn period, the chest roentgenogram is generally normal. As pulmonary vascular resistance drops during the 1st several weeks of life, evidence of increased pulmonary blood flow becomes apparent. Arterial PO2 is low and does not rise appreciably after the patient breathes 100% oxygen (hyperoxia test), although this test may not be totally reliable. Echocardiography is diagnostic and confirms the transposed ventricular-arterial connections (Fig. 425-2). The size of the interatrial communication and the ductus arteriosus can be visualized and the degree of mixing assessed by pulsed and color Doppler examination. The presence of any associated lesion, such as left ventricular outflow tract obstruction or a VSD, can also be assessed. The origins of the coronary arteries can be imaged, although echocardiography is generally not as accurate as catheterization for this purpose. Cardiac catheterization may be performed in patients for whom noninvasive imaging is diagnostically inconclusive, where an unusual coronary artery anomaly is suspected, or in patients who require emergency balloon atrial septostomy. Catheterization will show right ventricular pressure to be systemic because this ventricle is supporting the systemic circulation. The blood in the left ventricle and pulmonary artery has a higher oxygen saturation than that in the aorta. Depending on the age at catheterization, left ventricular and pulmonary arterial pressure can vary from systemic level to <50% of systemic-level pressure. Right ventriculography demonstrates the anterior and rightward aorta originating from the right ventricle, as well as the intact ventricular septum. Left ventriculography shows that the pulmonary artery arises exclusively from the left ventricle.
Treatment
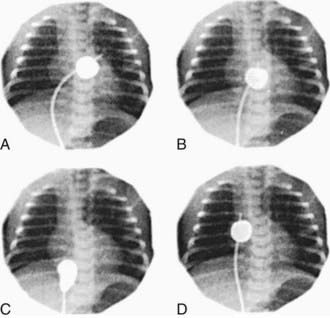
Infants who remain severely hypoxic or acidotic despite prostaglandin infusion should undergo Rashkind balloon atrial septostomy (Fig. 425-3). A Rashkind atrial septostomy is also usually performed in all patients in whom any significant delay in surgery is necessary. If surgery is planned during the 1st 2 wk of life, and the patient is stable, catheterization and atrial septostomy may be avoided.
A successful Rashkind atrial septostomy should result in a rise in PaO2 to 35-50 mm Hg and elimination of any pressure gradient across the atrial septum. Some patients with TGA and VSD (Chapter 425.3) may require balloon atrial septostomy because of poor mixing, even though the VSD is large. Others may benefit from decompression of the left atrium to alleviate the symptoms of increased pulmonary blood flow and left-sided heart failure.
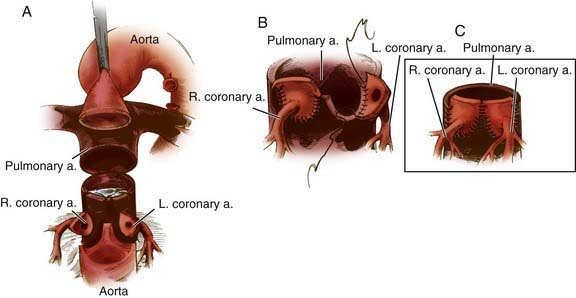
The arterial switch (Jatene) procedure is the surgical treatment of choice for neonates with d-TGA and an intact ventricular septum and is usually performed within the 1st 2 wk of life. The reason for this time frame is that as pulmonary vascular resistance declines after birth, pressure in the left ventricle (connected to the pulmonary vascular bed) also declines. This drop in pressure results in a decrease in left ventricular mass over the 1st few weeks of life. If the arterial switch operation is attempted after left ventricular pressure (and mass) has declined too far, the left ventricle will be unable to generate adequate pressure to pump blood to the high pressure systemic circulation. The arterial switch operation involves dividing the aorta and pulmonary artery just above the sinuses and re-anastomosing them in their correct anatomic positions. The coronary arteries are removed from the old aortic root along with a button of aortic wall and reimplanted in the old pulmonary root (the “neoaorta”). By using a button of great vessel tissue, the surgeon avoids having to suture directly onto the coronary artery (Fig. 425-4); this is the major innovation that has allowed the arterial switch to replace previous atrial switch operations for d-TGA. Rarely, a two-stage arterial switch procedure, with initial placement of a pulmonary artery band, may be used in patients presenting late who already have had a reduction in left ventricular muscle mass and pressure.
425.4 L-Transposition of the Great Arteries (Corrected Transposition)
The physiology of l-TGA is quite different from that of d-TGA. Desaturated systemic venous blood returns via the vena cavae to a normal right atrium, from which it passes through a bicuspid atrioventricular (mitral) valve into a right-sided ventricle that has the architecture and smooth wall morphologic features of the normal left ventricle (Fig. 425-5). Because transposition is also present, however, the desaturated blood ejected from this left ventricle enters the transposed pulmonary artery and flows into the lungs, as it would in the normal circulation. Oxygenated pulmonary venous blood returns to a normal left atrium, passes through a tricuspid atrioventricular valve into a left-sided ventricle, which has the trabeculated morphologic features of a normal right ventricle, and is then ejected into the transposed aorta. The double inversion of the atrioventricular and ventriculoarterial relationships result in desaturated right atrial blood appropriately flowing to the lungs and oxygenated pulmonary venous blood appropriately flowing to the aorta. The circulation is thus physiologically “corrected.” Without other defects, the hemodynamics would be nearly normal. In most patients, however, associated anomalies coexist: VSD, Ebstein-like abnormalities of the left-sided atrioventricular (tricuspid) valve, pulmonary valvular or subvalvular stenosis (or both), and atrioventricular conduction disturbances (complete heart block, accessory pathways such as Wolff-Parkinson-White syndrome).
Beca J, Gunn J, Coleman L, et al. Pre-operative brain injury in newborn infants with transposition of the great arteries occurs at rates similar to other complex congenital heart disease and is not related to balloon atrial septostomy. J Am Coll Cardiol. 2009;53:1807-1811.
Bellinger DC, Newburger JW, Wypij D, et al. Behaviour at eight years in children with surgically corrected transposition: The Boston Circulatory Arrest Trial. Cardiol Young. 2009;19:86-97.
Bellinger DC, Wypij D, duDuplessis AJ, et al. Neurodevelopmental status at eight years in children with dextro-transposition of the great arteries: The Boston Circulatory Arrest Trial. J Thorac Cardiovasc Surg. 2003;126:1385-1396.
Blume ED, Wernovsky G. Long-term results of arterial switch repair of transposition of the great vessels. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 1998;1:129-138.
Dearani JA, Danielson GK, Puga FJ, et al. Late results of the Rastelli operation for transposition of the great arteries. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2001;4:3-15.
Dunbar-Masterson C, Wypij D, Bellinger DC, et al. General health status of children with D-transposition of the great arteries after the arterial switch operation. Circulation. 2001;104(Suppl 1):I138-I142.
Hayes CJ, Gersony WM. Arrhythmias after the Mustard operation for transposition of the great arteries: a long-term study. J Am Coll Cardiol. 1986;7:133-137.
Khairy P, Van Hare GF. Catheter ablation in transposition of the great arteries with Mustard or Senning baffles. Heart Rhythm. 2009;6:283-289.
Ly M, Belli E, Leobon B, et al. Results of the double switch operation for congenitally corrected transposition of the great arteries. Eur J Cardiothorac Surg. 2009;35:879-883.
Neufeld RE, Clark BG, Robertson CM, et al. Five-year neurocognitive and health outcomes after the neonatal arterial switch operation. J Thorac Cardiovasc Surg. 2008;136:1413-1421.
Pedra SR, Pedra CA, Abizaid AA, et al. Intracoronary ultrasound assessment late after the arterial switch operation for transposition of the great arteries. J Am Coll Cardiol. 2005;45:2061-2068.