Chapter 657 Cutaneous Bacterial Infections
657.1 Impetigo
Etiology/Pathogenesis
Bullous impetigo is always caused by S. aureus strains that produce exfoliative toxins. The staphylococcal exfoliative toxins (ETA, ETB, ETD) blister the superficial epidermis by hydrolyzing human desmoglein 1, resulting in a subcorneal vesicle. This is also the target antigen of the autoantibodies in pemphigus foliaceus (Chapters 174 and 176).
Clinical Manifestations
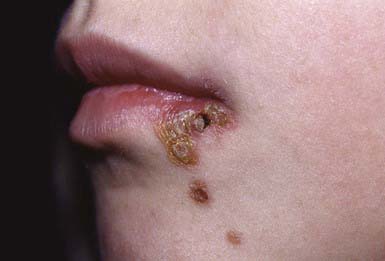
Nonbullous Impetigo
Nonbullous impetigo accounts for > 70% of cases. Lesions typically begin on the skin of the face or on extremities that have been traumatized. The most common lesions that precede nonbullous impetigo are insect bites, abrasions, lacerations, chickenpox, scabies pediculosis, and burns. A tiny vesicle or pustule forms initially and rapidly develops into a honey-colored crusted plaque that is generally <2 cm in diameter (Fig. 657-1). The infection may be spread to other parts of the body by the fingers, clothing, and towels. Lesions are associated with little to no pain or surrounding erythema, and constitutional symptoms are generally absent. Pruritus occurs occasionally, regional adenopathy is found in up to 90% of cases, and leukocytosis is present in about 50%.
Complications
Infection with nephritogenic strains of GABHS may result in acute poststreptococcal glomerulonephritis (Chapter 505.1). The clinical character of impetigo lesions is not predictive of the development of poststreptococcal glomerulonephritis. The most commonly affected age group is school-aged children, 3-7 yr old. The latent period from onset of impetigo to development of poststreptococcal glomerulonephritis averages 18-21 days, which is longer than the 10-day latency period after pharyngitis. Poststreptococcal glomerulonephritis occurs epidemically after either pharyngeal or skin infection. Impetigo-associated epidemics have been caused by M groups 2, 49, 53, 55, 56, 57, and 60. Strains of GABHS that are associated with endemic impetigo in the USA have little or no nephritogenic potential. Acute rheumatic fever does not occur as a result of impetigo.
Bernard P. Management of common bacterial infections of the skin. Curr Opin Infect Dis. 2008;21:122-128.
Chen AE, Carroll KC, Diener-West M. Randomized controlled trial of cephalexin versus clindamycin for uncomplicated pediatric skin infections. Pediatrics. 2011;127(3):e573-e580.
Parish LC, Parish JL. Retapamulin: a new topical antibiotic for the treatment of uncomplicated skin infections. Drugs Today. 2008;44:91-102.
Schachner LA. Treatment of uncomplicated skin infections in the pediatric and adolescent patient populations. J Drugs Dermatol. 2005;4:s30-s33.
657.2 Subcutaneous Tissue Infections
Necrotizing Fasciitis
Etiology
The rest of the cases and the most fulminant infections, associated with toxic shock syndrome and a high case fatality rate, are usually caused by S. pyogenes (Chapter 176). Streptococcal necrotizing fasciitis may occur in the absence of toxic shock–like syndrome and is potentially fatal and associated with substantial morbidity. Necrotizing fasciitis can occasionally be caused by S. aureus; Clostridium perfringens; Clostridium septicum; P. aeruginosa; Vibrio spp., particularly Vibrio vulnificus; and fungi of the order Mucorales, particularly Rhizopus spp., Mucor spp., and Absidia spp. Necrotizing fasciitis has also been reported on rare occasions to result from non–group A streptococci such as group B, C, F, or G streptococci, S. pneumoniae, or H. influenzae type b.