Despite how “uncomplicated” a birth may seem, the physical trauma of that event may have its own peculiar dangers to the cranial and spinal structures of the infant (
1,
2,
3). These injuries, if left unidentified and unattended, may adversely affect the physical, emotional, and mental growth and development of the child during one of the most vital periods of his or her life. Identifying and addressing the resultant injuries may be a large challenge, especially to the uninitiated. Those who choose to accept the challenge are advised to acquire the knowledge and skills necessary to address both spinal and cranial injuries. This chapter will be limited to the cranial evaluation and will provide the reader with baseline information that may be used as a tool for those who wish to incorporate craniosacral therapy (CST) into their own treatment protocol or as knowledge to be used when a referral to a specialist is more appropriate.
Cranial adjusting procedures have been a part of chiropractic and osteopathic therapeutic repertoires for more than 60 years, and a small but growing body of literature generated during that time supports the concepts of cranial adjusting (
1,
2,
3,
4,
5,
6,
7,
8,
9,
10,
11,
12,
13,
14,
15,
16,
17,
18,
19). Though many within these two professions have investigated and reported on the effectiveness of cranial therapy, literature describing the efficacy of these practices during the perinatal period is limited and requires further investigation.
This chapter discusses the role of CST during the period from birth through the first year of life. This is a critical period of susceptibility, when craniosacral strain patterns may contribute to aberrant neuromusculoskeletal (NMS) growth and development. Adopting a “wait and see” attitude when an infant presents with possible NMS dysfunction is to allow developmental anomalies of the central nervous system to manifest as a result of an alteration of the basilar bones and membranes of the cranium. For the experienced practitioner who is familiar with the cranial concept and who has the palpatory skills necessary to apply CST during this period, each case will present with challenges and rewards. For others who are less experienced, treatment may include a referral to a qualified craniosacral practitioner.
Although there are many forms of cranial techniques taught within both the chiropractic and osteopathic professions, it is this author’s opinion that cranial treatment of the pediatric patient should be limited primarily to a nonforce indirect method. The forces of nature alone can cause considerable damage without additional forces being applied by well-meaning health care providers either during the birth process or afterward.
CST, as taught by the Upledger Institute, has two very broad goals: first, to improve the patient’s level of wellness by restoring optimum cerebrospinal fluid (CSF) physiology, and, secondly, by restoring balance to the reciprocal membranous tension within the cranial system, thus improving neurological function. There are also five very specific goals:
To reduce articular restrictions
To reduce membranous restriction patterns
To improve circulation
To reduce the potential for neural entrapment from exit foramen in the cranial base
To increase the vitality of the cranial rhythmic impulse (CRI) (
16)
LITERATURE
Research related to the cranial concept began in the 1960s and has focused primarily on the validation of motion within the cranial sutures and the palpable CRI. With the technological advances seen in the 1980s and 1990s, objective evidence has demonstrated that the adult sutures are in motion and are capable of being influenced by both internal and external forces. Though there is little doubt that the pediatric skull continues to mold and change its inherent shape well into adulthood,
it is helpful to review the most pertinent research as it relates to the validation of the cranial motion concept.
In 1970, an attempt was made to demonstrate cranial restriction patterns roentgenographically. Greenman (
20) found a strong correlation between roentgenographic findings and clinical observations made independently by a physician experienced with the cranial concept. It seemed that it was possible to demonstrate side bending, torsion, flexion, and extension patterns of the skull. Occasionally, lateral and vertical strain of the sphenobasilar (SB) junction also was demonstrated. Correlation of clinical observations with the finding of low occiput on the side of the low sacrum was excellent, but that with the lumbosacral (LS) angle and the angle of the base of the skull was extremely poor.
The hypothesis that the cranial bones move and possess a slow rhythmic motion pattern was investigated by a team of researchers at Michigan State University College of Osteopathic Medicine in 1975 by direct instrumentation of cranial bone displacement in the monkey. Parietal bone displacement patterns were recorded. One corresponded to the respiratory frequency; another of 5 to 7 cycles per minute corresponded to neither heart rate nor changes in central venous pressure. This was later identified as the CRI (
21).
In 1977, Upledger (
22) reported on the interexaminer reliability of cranial examination findings. The examinations were performed on schoolchildren ages 3 to 5 years by skilled examiners. An analysis of the data (
Table 34.1) derived from the 50 craniosacral examinations on the 25 preschool children indicated high levels of agreement (<0.80) on the reproducibility of their findings. The study also helped to establish the CRI as an independent physiologic rhythm.
Upledger (
23) reported in 1979 on a study of mechanoelectric measurements performed on patients that showed distinct strain gauge, electrocardiography, electromyography, and integrated electromyography patterns. These patterns corresponded with palpatory sensations perceived by cranially oriented physicians. This correlation far exceeded random probability. This study demonstrated that subjective impressions of various changes in the craniosacral mechanics, which are reported by a trained craniosacral practitioner, are documentable by objective instruments.
Pederick (
4) reported in 1984 on studies conducted to investigate the CRI. Computerized tomography scans were performed to follow up on telemetry and ultrasound studies that showed brain motion coincident with sinusoidal pressure waves at a frequency of 2 to 9 cycles per minute. The examiners were able to detect even lower frequency waves, suggesting complex peristaltic movement of the brain powered by the flow of blood and CSF through the ventricular cisternal system.
In 1991, Kostopoulos and Keramidas (
24) reported on a cadaver study that attempted to validate the mobility of cranial sutures. The study suggested that, when a controlled external force was applied to the frontal bone of an embalmed cadaver, the force would be transmitted to the falx cerebri, resulting in a relative elongation of that membrane. There was a positive but low correlation between the applied force and the relative elongation of the falx cerebri. This offers validation to the scientific basis of CST and supports the contention that cranial sutures are mobile even after death.
In 1992, an instrument was developed by the research team at the University of Michigan College of Osteopathy to quantify parietal bone motion. The isotonic measuring device was attached to the surgically exposed skulls of anesthetized adult cats in an attempt
to measure the bidirectional cranial bone motion relative to the skull’s sagittal suture. The resulting data, which was reported by Adams et al. (
25) in 1992, indicated that not only did the parietal bones move in reference to one another by forces applied externally to the head, they also were displaced by intracranial forces. CSF pressure increases accompanying induced hypercapnia or injected norepinephrine were closely correlated with lateral, if not rotational, parietal bone movement during both onset of effect and recovery from it. Similar correspondence was demonstrated when intracranial pressure was increased by bolus injections of artificial CSF.
Pick (
14) investigated the effects of external maxillary and frontal parietal manipulation on intracranial structures of the human brain and reported his findings in 1994. A 42-year-old man participated as the subject in the intriguing experiment. Magnetic resonance imaging was used to evaluate the internal cranial structures while one investigator applied a light pressure to the subject’s parietal/frontal area and a second investigator applied light pressure to the subject’s hard palate. A scan of the midsagittal scan was obtained while the investigators touched the contact points, and a second scan was made while the investigators applied the appropriate pressure to the contact areas. Gross changes in the brain’s internal structures were visible after the application of the external manipulation. The study lends support to the theory that external cranial manipulation causes detectable changes in the cranial vault.
During the last two decades, several investigators have conducted large-scale clinical trials with newborns and small children in an attempt to validate the correlation between cranial and spinal restriction patterns and neurophysiological findings.
In 1992, Biedermann (
2) reported on a study in which he used several thousand case histories and catamneses to investigate the pathogenetic potential of the craniovertebral junction among newborns and young children. He reported that manual therapy cannot be the universal remedy for all children suffering from kinematic imbalances caused by suboccipital strain, but it should be considered as one of the most efficient therapies available.
Frymann (
3) conducted a retrospective study of several hundred children to investigate the correlation between a distinctive traumatic pattern within the craniosacral mechanism and subsequent learning problems. She discovered that 72.8% of infants in whom learning problems later developed had suffered some considerable trauma before or during birth compared with 28.3% of those without learning problems.
Upledger (
26) conducted a standardized craniosacral examination on a mixed sample of 203 grade-school children. The probabilities calculated supported the existence of a positive correlation between elevated craniosacral motion restriction scores and subsequent disabilities.
CLINICAL ANATOMY
The use of CST requires that the practitioner conduct an intense academic study of the osseous cranium, sutures, and meninges. For application during the perinatal period, the practitioner also is encouraged to conduct a study of the process of birth so that the presentation of abnormal structure and/or function can be addressed accurately. Though it is not within the scope of this chapter to provide such detailed information, an attempt will be made to summarize the most important aspects of newborn anatomy and consequences of birth trauma.
Dural Membranes and Septa
Falx Cerebri A vertical sheet of tough, relatively elastic connective tissue in the newborn, the falx cerebri arises from both the occipital and the frontal bones. This membrane contributes to the interior ceiling cover and separates the cortical hemispheres as it affords passage for the sagittal and inferior venous sinuses (
Fig. 34-1). It is not difficult to imagine abnormal membrane tensions interfering with normal cranial bone motion and with free blood flow through this venous sinus system, which transmits tension from any source through itself in a direction dictated by its geometry and its attachments (
16).
Tentorium Cerebelli The superior leaves of the tentorium cerebelli (TC) are continuous with the two layers of the falx cerebri as they separate to form the two superior and horizontal walls of the straight venous sinus (
Fig. 34-1). The inferior layers of the leaves of the TC are continuous with the inferior walls of the straight sinus and then with the two layers of the falx cerebelli. The inferior layers attach anteriorly to the posterior clinoid processes, and the superior layers attach to the anterior clinoid processes of the sphenoid bone. Lateral to the clinoid attachments, both leaves of the TC attach to the petrous ridges of the temporal bone. Here the tentorium encloses the superior petrosal sinuses.
Moving posteriorly, the TC attaches to the mastoid portions of the temporal bones, then to the parietal bones, and, finally, to the transverse ridges of the occiput, where it affords passage to the transverse venous sinuses. Clinically, rotational dysfunction of the temporal bones may be related etiologically to many visual motor problems. This occurs because
rotational dysfunction of the temporal bone places increased tension into the anterior cerebellar tent at the petrous ridges. Cranial nerves III, IV, V, and VI all pass between the leaves of the TC. Correction of the anatomicophysiological dysfunction of the temporal bone often rapidly and dramatically corrects strabismus and nystagmus (
16).
Falx Cerebelli The falx cerebelli houses the occipital venous sinus and forms the tough fibrous ring around the foramen magnum (FM). Tension upon this falx can interfere with the free passage of venous blood through that sinus. The falx cerebelli, TC, and spinal dural tube may all transmit tension to the FM and surrounding tissue. This tension, along with cervical muscle hypertonus, somatic dysfunction of the occipital condyles, and cranial base dysfunction, may interfere with normal function of structures within the jugular foramina (
Fig. 34-1). This results in intracranial fluid congestion caused by venous back pressure, and symptoms result from dysfunction of cranial nerves IX, X, XI, and XII (
27).
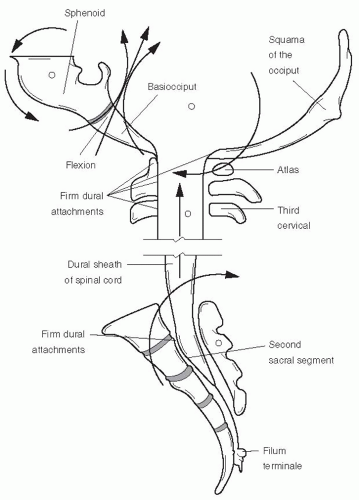
Spinal Dura Relatively elastic in the infant, the spinal dura mater attaches firmly at the FM, to the posterior bodies of C2 and C3, and to the body of the S2 sacral segment, where it becomes the filum terminale externus; passes out the sacral hiatus; and attaches to the coccyx as its periosteum (
Fig. 34-2). The endosteal layer of the intracranial dura mater is represented below the FM by the vertebral periosteum, which lines this canal. It is considered to be an extension of the inner or meningeal layer of the intracranial dura mater (
16). With each pair of spinal nerves is a duplication of the dura, which partly fuses with the periosteum at the intravertebral foramen. Thus, any segmental lesion may adversely affect the function of the craniosacral system.
Newborn Cranium
Vault Bones The newborn skull is extremely fragile and has no bony sutures. The bones, particularly of the vault, are still floating in membrane, which provides flexibility during the molding process. The bones in the base of the skull develop in cartilage. This provides a stronger and more protective support for the brain as the fetus
maneuvers the birth canal. The frontal bone develops in two halves. Its midline suture is obliterated by the eighth year, but it may persist (among some races more often than others) and is then known as the metopic suture. At birth the mastoid process is still undeveloped, thus exposing both the styloid process and the stylomastoid foramen. Compression or distortion of this foramen may result in facial nerve dysfunction.
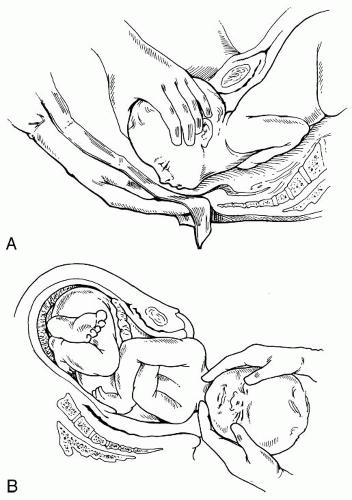
The temporal and sphenoid each consist of three membranous segments, which will continue to develop and unite during the first year of life. Excessive force applied to these structures during pregnancy, labor, or assisted delivery (i.e., forceps, vacuum, and/or the Ritgen Maneuver) (
Fig. 34-3A,B) may distort the membranous segments and alter the function of the associated soft tissue. This may go unnoticed until the infant exhibits any number of symptoms, one of which may be chronic otitis media with hypertonicity or hypotonicity of the tensor tympany and tensor veli palatine muscles. This may result in persistent retraction and eustachian tube patency.
The occiput consists of four segments: the squama, two condylar parts, and the basilar portion. The occipital squama unites with the condylar segments between 3 and 5 years of age. The condylar segments unite with the basilar portion between 7 and 8 years of age. The occiput directs the pattern of the cranium while the sphenoid directs the pattern of the facial bones. If during development there is a malalignment or crowding of these segments, developmental deformity of the cranial base will result. This deformity may be obvious through excessive molding and craniofacial deformities, or it may go unnoticed until the patient suffers serious sequelae to some minor injury. If severe, a spinal scoliotic compensation may develop (
1,
13,
16).
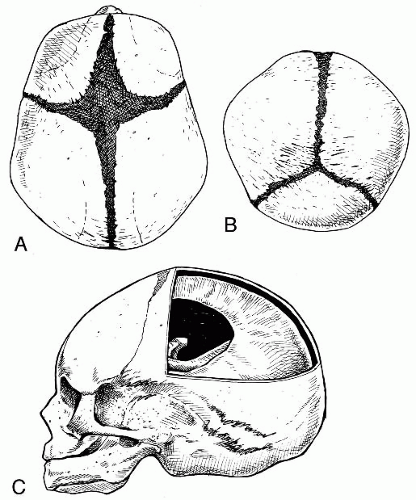
Fontanelles The anterior, posterior, sphenoidal, and mastoid fontanelles are membranous gaps of dura mater formed at the corners of the parietal bones (
Fig. 34-4A-C). The anterior fontanelle is the largest of the membranous gaps, which are literally small springs, or fountains, that fluctuate with changes in intracranial pressure. At birth the brain is 25% of its adult size and will grow to 75% of its full size by the end of the first year. The fontanelles located at the bregma, lambda, pterion, and asterion allow for this growth. As the cranial bony plates continue to ossify on and across the dura mater, the fontanelles eventually will reduce in size until the cranial plates join to form the moveable cranial sutures.
Sphenobasilar Articulation The most important of the cranial articulations, the SB synchondrosis is formed at the juncture between the occiput and the sphenoid (
Fig. 34-5A). During the birth process and with the initiation of breathing, the SB plate, consisting largely of cancellous bone surrounded by a very thin layer of dense bone, is capable of flexing in various directions. Anatomical distortions between the components of the SB synchondrosis are secondary to cranial base suture dysfunctions and/or abnormal membrane tensions within the dura mater. Therefore, after delivery, the continued flexibility depends on (a) the freedom of motion of the petrous portions of the temporal bones and associated sutures, and (b) the lack of tension in the dural stress fibers about the base of the skull and throughout the vertebral canal (
16) (
Fig. 34-5B).
The SB and LS articulations are in the same plane and may move synchronously to initiate and regulate
the rate of the CRI (
6,
7,
16). During inhalation, both articulations are moved physiologically into flexion about a transverse axis (
Fig. 34-6). At the same time, the paired bones of the periphery are rotated externally. Extension and internal rotation follow during the exhalation phase. Sutural restrictions, soft tissue distortions, segmental lesions within the skeletal system (see
Chapter 15), and imbalance within the reciprocal membranes of the dura mater may all influence the SB and LS articulations as they move in synchrony (
14,
16,
28) (
Figs. 34.7 and
34.8).
Gestation Compressive forces applied to the fetal spine and cranium during the last weeks of pregnancy begin the cranial molding process and prepare the fetus for descent through the birth canal. These forces are influenced by the strength of Braxton Hicks contractions and the degree of maternal pelvic and perineal resistance. In-utero cranial molding is also influenced by the position of the fetus in relation to the placenta, the maternal spine and its degree of lordosis, the inclination and mobility of the maternal sacrum, contour of the bony pelvis, and the innervation of uterine musculature.
In-Utero Constraint
Confining counteractive forces, such as those that occur with constraint or compartment syndromes, alter the plane of the temporal bone and thus alter the tensity of the dura. The force applied to the stress fibers of the dural membranes may then alter (a) the pattern of the cranial base at the craniofacial junction, thus altering the plane of the face, and (b) the craniovertebral junction, thus altering the plane of the sacrum. Should these forces persist throughout labor and delivery, excessive molding may occur. Reinforcing stress fibers within the dural membranes help to prevent severe overriding of the bones, which would result in tearing of the venous sinuses (
Fig. 34-5A,B).
Birth Process
During an uncomplicated, natural delivery process, certain basic tenets of childbirth occur during the last month of pregnancy to prepare both the mother and the fetus for delivery. First, there is an increase in maternal compressive force through the fetal spine and upon the cranial base. This leads to an alteration in the various planes of stress fibers throughout the fetal cranium. With each stage and change in position there will be stress placed upon a different plane of the protective intracranial dural membranes (
1). By this time, the long axis of the fetal spine ideally is parallel with the long axis of the uterus, and the short axis of the cranium ideally is perpendicular to the long axis of the fetal spine. This will allow for forward cervical flexion without rotation.
Second, with each ensuing contraction, fluid is forced out of the skull to reduce its size and to allow cranial molding to begin. This generally consists of the moderate overriding and compression that occur as the cranial segments meet the resistance of the sacral promontory and soft tissue within the pelvic floor. The flexible membranous cranial vault will correct itself after a few days if excessive force was not applied to any particular group of stress fibers.
After birth, crying balloons out the perimeter of the skull and suckling flexes the SB articulation via the vomer. Both of these newborn behaviors will normalize the pull of the intracranial membranes as the associated stress fibers become balanced within the skull. Thus, in an uncomplicated case, the general morphology,
symmetry, and mobility of the individual preosseous elements are retained (
13).
Birth Trauma Birth-related trauma may be defined as tissue deformation, distortion, disruption, or destruction sustained by the fetus. The origin of obvious birth trauma has been addressed in the literature and may be classified under three headings: (a) gestational, (b) incident to labor, and (c) trauma during delivery. These in turn are considered to be injuries to the brain, the cord, the peripheral nerves, and the musculoskeletal system. They are reported as:
Trauma to the bony/cartilaginous skeleton such as skull fractures (
29,
30,
31), skull depression (
32), and nasal trauma (
30)
Trauma to vascular tissue and fluid dynamics such as cephalhematomas (
29), retinal hemorrhages (
33,
34),
intracranial hemorrhages (
35,
36,
37,
38), intracranial arterial aneurysm (
39), extradural hemorrhages (
40), and hydrocephalus (
41)
Trauma to nervous tissue such as spinal cord injury (
29,
30,
42), Erb’s Palsy (
43), brachial plexus injury (
30), and ocular injuries (
30)
Trauma to soft tissue components such as congenital torticollis (
44,
45,
46,
47,
48,
49,
50,
51,
52,
53,
54,
55,
56,
57), intra-abdominal trauma (
29), and diaphragmatic paralysis (
30)
Asphyxia caused by anoxia or injudicious use of anesthetics (
58)
Asphyxia resulting in fetal mortality is said to be one of the most reported incidents of birth trauma (
58). Conversely, it has been reported that newborn brain stem, spinal cord, and other musculoskeletal injuries may be the most underreported causes of infant morbidity (
42). A skilled practitioner, therefore, should be prepared to
perform an evaluation that includes an assessment of all cranial and spinal structures.