Craniofacial Defects
Robert J. Gorlin
EMBRYOLOGY OF THE PRIMARY AND SECONDARY PALATES
The neural crest plays an integral part in facial morphology. When the neural folds fuse to form the neural tube at approximately the fourth week of gestation, ectomesenchymal cells adjacent to the neural plate migrate into the underlying regions. Those in the head and face form essentially all the skeletal and connective tissues of the face: bone, cartilage, fibrous connective tissue, and all dental tissues except enamel. This transformation is effected by induction of these ectomesenchymal cells by the adjacent oral ectoderm and pharyngeal endoderm. Neural crest cells also migrate into the visceral arches, where they surround mesodermal cores.
By the end of the fourth week, the anterior neuropore has closed. What is to be the face consists of a large frontal prominence overlying the first or mandibular arch. If one manually elevates the frontal prominence, one can see into the primary mouth or stomodeum. The primary mouth is separated from the foregut by the buccopharyngeal membrane, which undergoes programmed cell death and ruptures at approximately this time in development. On both sides of the frontal prominence, the nasal placodes are forming. These bilateral structures, located just above the primitive mouth, are represented by local thickening of the surface ectoderm. Rapid proliferation of tissue known as nasal swelling occurs both lateral and medial to the nasal placodes. By means of selective cell death and proliferation of tissues, nasal or olfactory pits that extend into the primitive mouth are formed; they are the primitive nostrils.
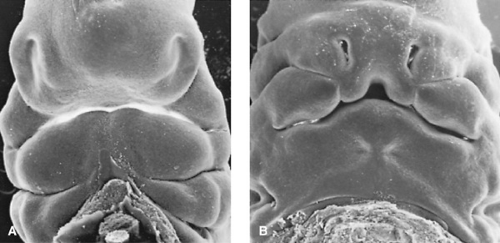
Extremely active growth occurs during the fifth and sixth weeks (Fig. 69.1). The maxillary swellings, which represent the upper portion of the first pharyngeal arch, enlarge considerably and, by pushing the nasal swellings or prominences medially, cause them to approach each other in the midline. When the two prominences meet, the median nasal prominences and the maxillary swellings merge. Thus, the upper lip is formed laterally by the maxillary prominences and medially by the fused median nasal prominences. This development occurs near the seventh week. The lateral nasal prominences play no role in formation of the upper lip but form the alae or wings of the nose.
The primary palate consists of the two merged medial nasal processes that form the intermaxillary segment. The intermaxillary segment consists of two portions: a labial component that forms the philtrum of the upper lip (i.e., the indented area flanked by roughly parallel ridges that run from the columella of the nose to the middle of the upper lip) and the triangular palatal component of bone (premaxilla) that includes the four maxillary incisor teeth. The primary palate extends posteriorly to the incisive foramen or, clinically, to the incisive papilla.
The so-called secondary palate forms at least 90% of the hard and soft palates (i.e., all except the anterior portion that holds the incisor teeth). Its development appears to be somewhat more complicated than originally was thought. The palatal shelves originate as swellings or shelflike burgeoning of the medial surfaces of the maxillary prominences. They appear in the sixth week and grow downward, lateral to and somewhat beneath the tongue. Elevation of the palatal processes to a horizontal plane is more “rigorous” anteriorly, nearer the primary palate. Elevation begins during the seventh week.
What promotes the elevation has been called intrinsic shelf force, but it has a complex biochemical and physiochemical basis. When the shelves are elevated to the horizontal plane, programmed cell death occurs in the overlying epithelium, permitting flow of ectomesenchyme from each side to close the gap. Complete fusion is effected by the tenth week (Fig. 69.2). In some infants, cystic degeneration of the epithelial remnants occurs, producing evanescent midline palatal microcysts.
CLEFT LIP AND CLEFT PALATE
Epidemiology and Genetics
The degree of cleft formation varies greatly. Minimal degrees of involvement include bifid uvula, linear lip indentations
[so-called intrauterine (incisive foramen)-healed clefts], and submucous palatal cleft. Clefts may involve only the upper lip or may extend to the nostril and may be combined with defects of the hard or soft palate. Isolated palatal clefts may be limited to the uvula or they may be more extensive, cleaving the soft palate or both the soft and hard palates to just behind the incisor teeth.
[so-called intrauterine (incisive foramen)-healed clefts], and submucous palatal cleft. Clefts may involve only the upper lip or may extend to the nostril and may be combined with defects of the hard or soft palate. Isolated palatal clefts may be limited to the uvula or they may be more extensive, cleaving the soft palate or both the soft and hard palates to just behind the incisor teeth.
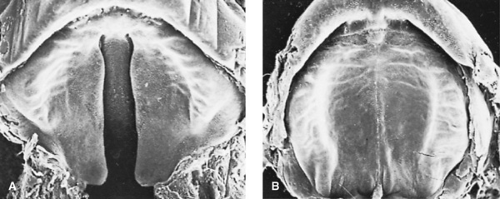 FIGURE 69.2. Scanning electron microscopy view of secondary palate at 8 weeks (A) and at 10 weeks (B). (Courtesy of K. Sulik, Chapel Hill, NC.) |
A combination of cleft lip and cleft palate is more common than isolated occurrences of either. Cleft lip with cleft palate composes approximately 50% of the cases, with cleft lip and isolated cleft palate each constituting perhaps 25%, generally irrespective of race. Cleft lip with or without cleft palate occurs in approximately 1 per 1,000 white births (range, 0.8 to 1.6 per 1,000). Frequency is higher in Native Americans (3.5 per 1,000), Japanese (2.1 per 1,000), and Chinese (1.7 per 1,000); it is lower among African Americans (0.3 per 1,000).
Isolated cleft lip may be unilateral (80%) or bilateral (20%). When unilateral, the cleft more commonly is located on the left side (approximately 70%), but it is no more extensive. Lips are cleft somewhat more frequently bilaterally (approximately 25%) when combined with cleft palate. The cleft lip and palate combination is more common in men than in women. Approximately 85% of cases of bilateral cleft lip and 70% of cases of unilateral cleft lip are associated with cleft palate. Cleft lip is not always complete (i.e., extending into the nostril). In approximately 10% of the cases, the cleft is associated with skin bridges or Simonart bands.
Isolated cleft palate appears to be an entity separate from cleft lip with or without cleft palate. Numerous investigators have determined that siblings of patients with cleft lip with or without cleft palate have an increased frequency of the same anomaly but not of isolated cleft palate, and vice versa. The incidence of isolated cleft palate among both Caucasians and African Americans appears to be 1 per 2,000 to 2,500 births. It occurs somewhat more often in girls, comprising approximately 60% of the cases. Although a 2:1 female-to-male predilection prevails for complete clefts of the hard and soft palate, the ratio approaches 1:1 for clefts of the soft palate only.
Cleft uvula varies in degree of completeness; incomplete clefts are more common. The frequency of cleft uvula (1 in 80 white persons) is much higher than that for cleft palate with no gender predilection. The frequency in parents and siblings of probands ranges from 7% to 15%. Cleft uvula among Native American groups is high, occurring in 1 per 9 to 14 births, depending on tribal group. In African Americans, it is rare. Estimates are 1 per 350 to 400 births.
Congenital velopharyngeal inadequacy, characterized by cleft palate speech (50%) without an overt cleft, is due to a short soft palate (60%), an imperfect muscular union across the soft palate (submucous palatal cleft), or increased depth of the nasal pharynx. Submucous palatal cleft is relatively uncommon, occurring in 1 in 1,200 children. Apparently no gender predilection is demonstrated. Approximately 30% of those with submucous palatal cleft have bifid uvula, with poor mobility demonstrated in 20%. A median deficiency or notch is seen in the bone at the posterior edge of the hard palate. It can be detected digitally or by a light probe placed within the nose. The occult submucous cleft palate can be detected via nasoendoscopy.
Recurrence data do not suggest a simple pattern of inheritance. This finding is bolstered by twin studies indicating the relative roles played by genetic and nongenetic influences. Among twins with cleft lip with or without cleft palate, concordance is far greater in monozygotic (35.0%) than in dizygotic (4.5%) twins. In twins with isolated cleft palate, concordance is not quite as great (monozygotic, 26.0%; dizygotic, 5.8%). This finding suggests a stronger genetic basis for cleft lip with or without cleft palate than for isolated cleft palate. Both cleft lip with or without cleft palate and isolated cleft palate consist of three groups: sporadic (75% to 80%), familial (10% to 15%), and syndromal (1% to 5%). Clefting is heterogeneous. Its variation and liability probably are determined by major genes, minor genes, environmental insults, and a developmental threshold.
Mechanisms of Cleft Production
By definition, cleft lip involves the failure of closure of the primary palate, and cleft palate involves failure of closure of the secondary palate. Knowledge regarding mechanisms involved in regulation of embryonic growth is sparse at best. Growth patterns can be affected also by environmental factors. A long list of teratogenic substances (e.g., corticosteroids, vitamin A, phenytoin, various folic acid antagonists) can produce clefting in rodents. Little evidence, however, suggests that any of these agents plays even a minor role in cleft palate production in humans.
Various genetic and environmental factors may inhibit the flow of neural crest cells or may affect their volume or mass so that contact between prominences is impossible or inadequate. The epithelium covering the ectomesenchyme may not undergo programmed cell death, so fusion cannot take place. Exact timing and exact positioning play critical roles.
Clefts of the primary and secondary palates occur in association in perhaps one-half of cases. A common mechanism of production has been sought. Reduction in size of both the labial maxillary prominence and the palatine process of the maxillary prominences appears to be a reasonable explanation.
Clefts of the secondary palate probably result from either hypoplasia of the shelves or delay in timing of shelf elevation. Experiments carried out on susceptible strains of mice suggest that both mechanisms are operative but at different times in
gestation. Large doses of vitamin A given to rodents early in gestation inhibit palatal shelf growth and cortisone given later in gestation inhibits palatal shelf elevation.
gestation. Large doses of vitamin A given to rodents early in gestation inhibit palatal shelf growth and cortisone given later in gestation inhibits palatal shelf elevation.
TABLE 69.1. FACIAL CLEFTS: RISK OF RECURRENCE | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Risk of Recurrence
In most cases, the cleft is either isolated or associated with a constellation of anomalies that do not form a recognizable syndrome. Although more than 300 cleft syndromes or associations are recognized, they constitute a low percentage of cases. Efforts must be made to recognize a cleft syndrome, because the pattern of inheritance may be simple, and the genetic risk for future affected children then may be more precise. For example, a parent with or without a cleft and paramedian pits of the lower lip has a 50% chance of having a child with cleft lip or palate.
In the case of isolated clefts, the risk to a first-degree relative of an affected individual is 2% to 4%. This information applies only to risks for similar anomalies (i.e., a parent with isolated cleft palate has no greater risk of having a child with cleft lip with or without cleft palate than anyone else, and vice versa). The risks increase as more individuals are affected. For example, if a parent and a child have clefts, the risk for a future affected sibling increases to approximately 10% to 12%. These and other situations are presented in detail in Table 69.1.
The severity of a facial cleft also affects recurrence risk in the offspring. For example, researchers have found that if a parent has isolated unilateral cleft lip, the recurrence risk is 2.5%. In the presence of unilateral cleft lip and palate, the risk increases to 4%, and the risk is more than 5.5% for bilateral cleft lip with cleft palate.
Care of the Infant with Cleft Lip or Cleft Palate
An interdisciplinary team—composed of a surgeon and a maxillofacial surgeon, orthodontist, audiologist, speech-language pathologist, prosthodontist, otolaryngologist, pediatric dentist, and geneticist—is extremely important in helping parents to understand the sequential approach to therapy for the many attendant problems.
Feeding usually requires considerable patience. Those with more severe clefts of the lip or palate should be fed in a sitting position to minimize fluid loss through the nose. Various techniques and equipment are used to feed infants with clefts, but no single method is optimal for all infants. Infants with cleft lip or cleft palate swallow normally but suck abnormally. A cleft in the lip or palate generally does not allow sufficient negative pressure. In the case of cleft lip only, breast-feeding or an artificial nipple with a large, soft base works well. For infants with cleft lip or palate, regular breast-feeding or normal bottle-feeding often is not successful because they are unable to seal either their lips or their velopharynx. With cleft of the palate only, breast-feeding or normal bottle-feeding usually can be carried out if the cleft is narrow or involves only the soft palate. Soft artificial nipples with large openings are more effective.
Regular bottle nipples do not work well for infants with wider palatal clefts or the Robin malformation sequence. Enlarging the nipple opening in association with a softer nipple with a large base and a long shaft often enables tongue movement to express a greater quantity of milk. One can also deliver milk directly into the mouth with a soft plastic bottle.
It is now well recognized that children with cleft palate will have increased frequency of middle ear infection and fluid resulting in conductive hearing loss. The tensor palatini muscle is the primary muscle that spans the Eustachian tube necessary for adequate ventilation of the middle ear spaces. In children with cleft palate, Eustachian tube dysfunction and subsequent negative pressure in the middle ear space is our ideal environment for fluid buildup and infection. If medication will not resolve chronic infections and fluid accumulation, myringotomies and placement of ventilation tubes are an effective treatment.
Tonsils and adenoids may be helpful in accomplishing adequate velopharyngeal closure for speech. Therefore, caution needs to be taken before considering their removal since velopharyngeal inadequacy and hypernasal speech may result with otoacoustic emission/auditory brainstem response testing.
Assessing auditory function in infants with cleft palates is important and may be carried out more accurately in infants or young children by auditory specialists.
Surgical Repair of Clefts
Closure of the lip usually is carried out between the second and tenth week after birth, depending on the infant’s weight and state of health. The primary purposes are to create a seal to allow normal sucking. Various techniques have been used for repair of the lip, depending on the degree and extent of defect. In those cases in which tissue in the two lip segments is insufficient to create an acceptable lip and nose, the surgeon may have to move small flaps of tissue from adjacent areas. For bilateral cleft lip, surgery is more difficult. If the primary palate is not attached to the secondary palate, it may require repositioning. Usually, subsequent surgery is required to correct nasal alar form, to compensate for uneven growth of tissue on the two sides of the lip, or to match evenly the vermilion line on both sides. This secondary surgery may best be performed during the teen years and is dependent upon the child’s physical and behavioral growth and development.
Surgical closure of the hard and soft palate often is performed at 9 to 12 months of age, but some surgeons prefer to wait longer. The object is to create airtight and fluid-tight closure of the cleft and to preserve the length and mobility of the soft palate, to accomplish adequate velopharyngeal closure.
Second surgery may be required to achieve adequate closure; sometimes a speech prosthesis may be the best approach.
Associated Anomalies
Cleft lip and palate often occur as isolated anomalies (i.e., thorough examinations conducted over several years have revealed no other primary abnormalities). This statement would
exclude, for example, the middle-ear infections that occur so frequently secondary to cleft palate.
exclude, for example, the middle-ear infections that occur so frequently secondary to cleft palate.
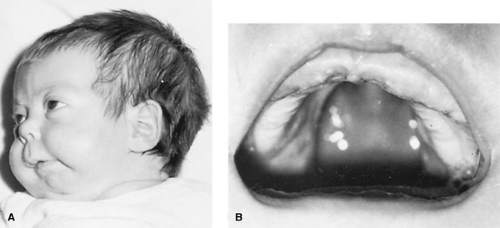 FIGURE 69.3. Robin malformation sequence. A: Small retruded lower jaw. B: U-shaped palatal cleft.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|