Orthotopic autotransplantation
Heterotopic autotransplantation
Site of transplantation
Pelvic
Extrapelvic
Prior stimulation of ovary required
No
No
Restoration of endocrine function
Yes
Yes
Ability for spontaneous pregnancy
Yes
No, requires IVF
Given that both of these techniques involve avascular grafting, there is a risk of post-grafting ischemia and follicular atresia. In fact, it has been shown that only 7% of the follicle loss is due to freezing/thawing process, while most of the follicles are lost due to ischemia/reperfusion injury [27]. One method to avoid the ischemia/reperfusion injury is autotransplantation of a frozen thawed whole ovary with its vascular pedicle back to the same patient [28]. It is a surgically challenging procedure, given difficult vascular and microvascular anastomoses, and has a higher risk of postoperative vascular complications that may compromise the entire ovary. Although live birth following autotransplantation of the whole ovary has been successfully shown in sheep, there are no human live births to date using autotransplantation of a whole ovary [29]. Although it remains a biologically plausible option, given clinical success rates of orthotopic and heterotopic autotransplantation, it may be reserved for rare circumstances [28].
28.5 Clinical Outcomes with Autotransplantation
The time to return of ovarian function following autotransplantation of frozen-thawed cortical ovarian tissue is varied in the literature but may resume 2–9 months postoperatively [9]. There is a varied rate of return of endocrine function, with two large meta-analyses reporting rates from 63.9% to 95%, respectively [6, 30]. The mean lifespan of the grafted tissue varies and can depend on the fraction of the ovary that was obtained, or the total surface area that was transplanted. The mean duration of the transplanted tissue is 4–5 years; however, there are reports lasting as long as 10 years to result in subsequent pregnancies [18].
In terms of its safety, there have been no reports in the literature of a recurrence of original cancer in correlation with orthotopic transplantation. Jadoul et al. were able to track surgical complications from 140 women who underwent OTC using responses of surveys and report four minor complications and one major complication resulting in reoperation for intra-abdominal hemorrhage. Patients reported a 96% satisfaction rate overall with the process. These data support the use of OTC, given its low complication and high satisfaction rates [31].
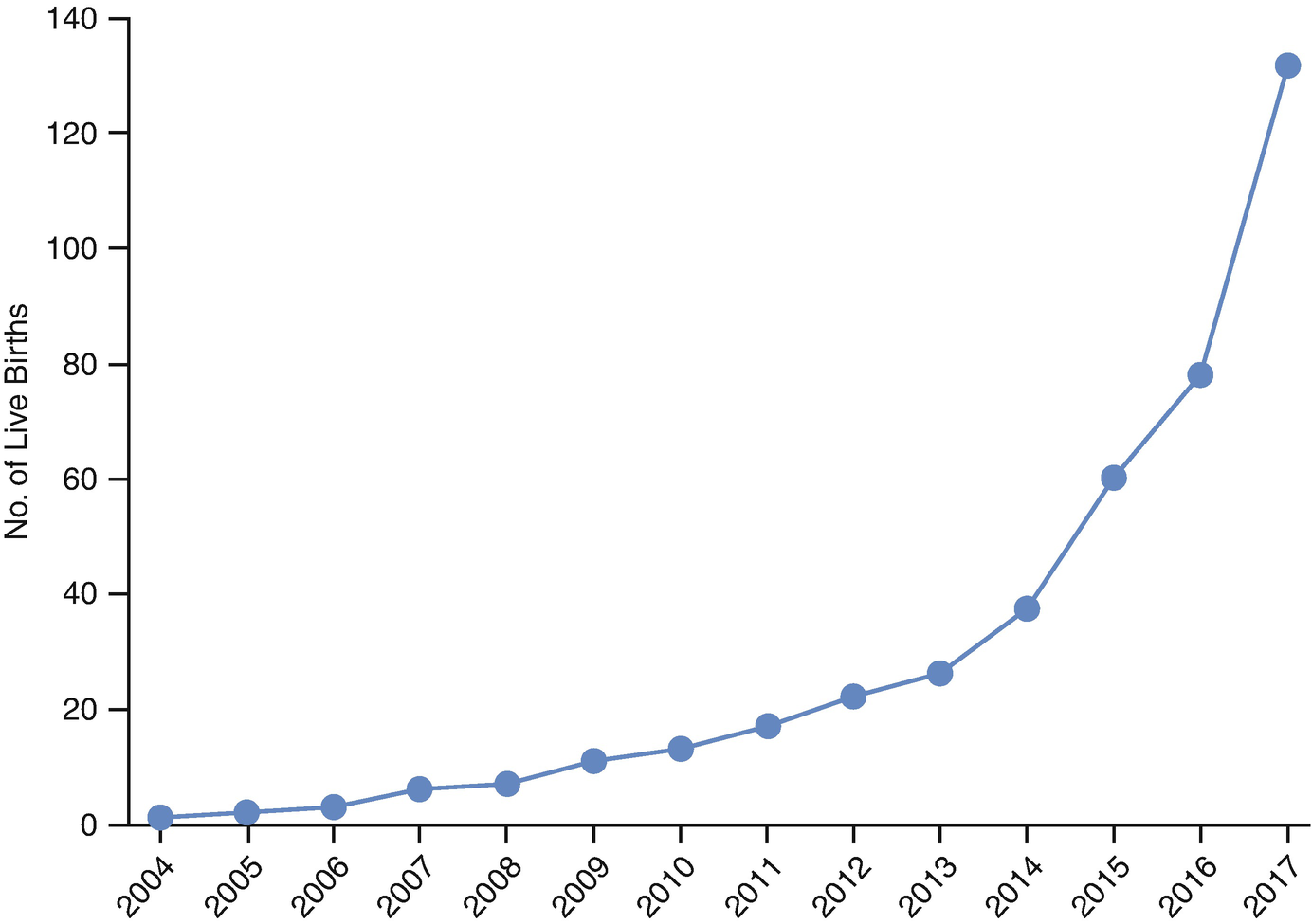
Live births following orthotopic autotransplantation from 2004 to 2017. (Donnez and Dolmans [7])
Neonatal outcome data of 40 of these live births have been reviewed and demonstrated that the mean gestational age at birth was 39 weeks and the mean weight at birth was 3168 g, consistent with other singleton pregnancies at birth. There has been only 1 fetal anomaly documented in 93 live births, which is consistent with the 1–2% rate of congenital anomaly in the general population [6, 18].
28.6 Limitations of Autotransplantation
While the success of autotransplantation has been revolutionary, these aforementioned methods have the limitation of carrying an additional risk of reintroducing malignant cells, particularly in instances of ovarian malignancies or in malignancies that may metastasize to the ovaries. Dolmans et al. used PCR-based studies to show malignant cell contamination of cryopreserved ovarian tissue from leukemia patients [36]. Despite evidence that frozen-thawed ovarian tissue with malignant contamination did not result in transmission of the disease xenografted to mice or that improvement in isolation technique involving washing the follicles three times showed no evidence of malignant cells, these methods are currently contraindicated in humans [37, 38]. Thus, in cases of leukemia or other cancers with high likelihood of ovarian involvement, alternative methods such as in vitro maturation (IVM) or ovarian follicle transplantation (artificial ovary) are needed.
28.7 In Vitro Maturation
IVM is a technique by which preantral follicles are extracted and isolated, and the final stages of maturation are completed in vitro in culture media. Immature oocytes progress from germinal vesicles in prophase I through meiosis I to reach metaphase II (MII). This stage of nuclear maturation, and additionally cytoplasmic maturation, need to occur in order to undergo fertilization and are under the control of epigenetic factors [39]. The first human live birth with this technique was documented in 1991; however, given overall lower success rates and the improvements of oocyte and embryo cryopreservation, the use of IVM has declined in clinical practice [40]. This method of fertility preservation, however, is favorable because it eliminates or reduces gonadotropin stimulation in the patient. This is particularly critical for those in which elevations in estradiol with stimulation are contraindicated, such as thromboembolism or hormone-sensitive malignancies. It also serves as an alternative for those who cannot delay gonadotoxic treatment. It can additionally be used for those with PCOS or those that are at a high risk of ovarian hyperstimulation syndrome.
There are two sources to obtain immature oocytes to employ the principles of IVM: [1] The first scenario involves a minimal controlled ovarian hyperstimulation (COH) or no prior COH, followed by aspiration of the ovarian follicles to obtain oocytes, and [2] the second scenario involves ovarian tissue freezing, thawing, followed by in vitro culture of the tissue for IVM, which also may include the use of an artificial ovary [41].
28.7.1 Obtaining Follicles for IVM via Aspiration
The process of oocyte aspiration has been described in IVM. Factors such as type of needle, aspiration pressures, or use of a mesh cell strainer often differ from protocols associated with oocyte retrieval in IVF given follicular sizes less than 10 mm in IVM [41]. Clinical outcomes for IVM for fertility preservation are promising although large prospective studies are lacking. In a large retrospective study of 192 IVM cycles using oocyte aspiration with no COH in women with cancer who require urgent chemotherapy, 105 IVM cycles (54.7%) resulted in cryopreservation of oocytes, and 82 IVM cycles (42.7%) resulted in cryopreservation of embryos. The results were comparable irrespective of the phase of the cycle in which oocyte aspiration occurred: early follicular, late follicular, or luteal [42]. When directly comparing oocyte aspiration with IVM to conventional IVF for fertility preservation in over 600 combined cycles, IVF had a statistically higher median number of oocytes collected and high rates of resultant oocyte and embryo cryopreservation. Of 33 cycles in which pregnancy was attempted, LBR per cycle was 31% following IVF and 7% following IVM, although these differences did not reach statistical significance [43].
28.7.2 Obtaining Follicles for IVM via OTC
Obtaining immature oocytes isolated from ovarian tissue has been described in the literature and can be followed by in vitro maturation and subsequent cryopreservation. In a small retrospective case series of four patients, the women underwent retrieval of immature oocytes from the antral follicles of the excised ovarian tissue, with a success of a total of eight mature oocytes vitrified [44]. This method led to an ongoing clinical pregnancy by Segers et al. in 2015, thus illustrating its clinical promise [45]. Sermondade et al. examined 54 patients who underwent oocyte vitrification after IVM associated with oocyte aspiration and compared primordial follicle density in OTC subsequently obtained in the same patient. This was an attempt to use antimullerian hormone (AMH) levels and antral follicle count (AFC) as clinical predictors of the number of oocytes cryopreserved after IVM. They deduced that an AMH and AFC of >3.5 ng/mL and >19 follicles, respectively, are required for obtaining at least eight frozen mature oocytes after IVM. They additionally found significant correlation of serum AMH with primordial follicles in the OTC sample. The primordial follicle density also correlated with the number of mature oocytes cryopreserved [46]. These data support the potential use of OTC as a method to obtain oocytes and the combined technique of follicle aspiration, followed by OTC, to increase chances of pregnancy. These clinical parameters may influence counseling of appropriate options of fertility preservation, although more studies are needed [47].
28.7.3 Artificial Ovary
The artificial ovary is a 3-D biocompatible and biodegradable matrix in which isolated follicles and ovarian stromal cells can be encapsulated and transplanted to patients. It serves as an important conduit for ovarian material because ovarian follicles are surrounded by a basal membrane, excluding them from the stromal environment, capillaries, and nerves. Isolation would then eliminate the risk of transmission of malignant cells during autotransplantation. Several research groups have contributed to both in vitro and animal in vivo studies [48–52]. The technique for developing an artificial ovary was based on the preliminary studies using suspension in plasma clots [53]. Studies in other fields had developed fibrin scaffolding as a matrix because it can facilitate cell proliferation, transplantation, and delivery of growth factors [54, 55]. This was applied to the ovary, where a fibrinogen droplet and the isolated ovarian stromal cells suspended in media were combined together. Thrombin was subsequently added to create the fibrin clot. In vitro studies showed two fibrin clot formulations had reproducible degradation of the fibrin network, had survival and proliferation of stromal cells, and had increasing stromal cell density [56]. This was then applied to a murine animal model with autotransplantation in a fibrin scaffold. Luyckx et al. showed that isolated murine preantral follicles survived and developed, supporting ovarian cells proliferated, and grafted endothelial cells proliferated with the formation of capillaries, all after 1 week of autotransplantation of fibrin matrices. Because of the successful fibrin matrix formulations, they recovered a greater number of follicles after autografting (32%), compared to 20.3% using human follicles grafted in a plasma clot in a prior study [53, 57]. Further murine studies showed secondary follicles were more likely to survive and develop than primordial primary follicles in a fibrin matrix after 1 week of grafting, which may imply that the composition of the fibrin matrix supports already existing larger follicles [58]. In 2016, Paulini et al. showed that isolated human follicles were able to survive after encapsulation in fibrin clots when xenografted in murine models [59]. Future studies are needed in this field using human follicles and transplantation, as this may be a favorable technique for fertility preservation.
Once the follicles are aspirated or isolated with either aforementioned method, they undergo maturation in vitro. There are several protocols describing maturation media; however, the addition of hormonal additives to the culture media seems to increase implantation rates [60]. FSH aids with expansion of the cumulus-oocyte complex (COC) and contributes to subsequent oocyte maturation, while LH and hCG facilitate resumption of meiosis and the final stages of maturation following what would be an LH surge and ovulation in vivo. Proteins are found in maturation media, although sources can vary from maternal serum and human follicular fluid to human serum albumin (HSA). Serum containing complex components including growth factors and amino acids may also be added [41].
To date, there have only been four live births as a result of IVM for fertility preservation in cancer patients; however, there are >1400 births as a result of IVM, with estimates >5000 births worldwide [43, 61]. Neonatal outcomes of children conceived using IVM has been described in the literature as being similar to conventional IVF [62–64]. A 2017 prospective single-blinded study compared outcomes at first trimester screening, 21 weeks of gestation, birth, and at 2 years of age and found no increased risks of offspring as a result of IVM compared to both IVF and ICSI controls [65]. IVM is still considered an experimental technique of fertility preservation, and more studies are needed to better elucidate the safety and efficacy as it is applied in clinical settings [66].
28.8 Future
Ovarian cortical tissue biopsy and freezing for autotransplantation has proven to be a valid method for fertility preservation, although it is still considered experimental. Future steps in this field should be taken to allow for broader clinical implementation. An international registry would support improved collection of data and tracking of clinical outcomes, with the eventual goal of allowing this technique to be no longer considered experimental. The need for developing selection criteria to guide clinical decision making has not been standardized, and a consensus on important factors may guide our ability to counsel patients on fertility preservation options. This technique boasts several advantages over currently used methods of oocyte and embryo cryopreservation, thus having the potential to provide a patient with more choices. Its shortcomings are attempted to be overcome using methods like IVM and the creation of an artificial ovary, although more research is needed to incorporate these techniques in routine clinical setting.

Full access? Get Clinical Tree


