MARIO M. LEITAO JR  CARMEN TORNOS
CARMEN TORNOS  AARON H. WOLFSON
AARON H. WOLFSON  ROISIN O’CEARBHAILL
ROISIN O’CEARBHAILL
Mesenchymal tumors of the uterine corpus are rare, accounting for approximately 7% to 8% of all uterine cancers (1). The outcomes of many of these tumors seem to be less favorable than many of the more common uterine carcinomas. However, outcomes do vary significantly based on the specific histology. This is a group of tumors felt to arise from the mesenchymatous portion of the uterine corpus and often considered as uterine “sarcomas.” The World Health Organization (WHO) classification of mesenchymal uterine corpus tumors is summarized in Table 23.1 (2). Uterine carcinosarcomas are probably not best classified as a uterine sarcoma or mesenchymal tumor any longer, but rather should be classified as a metaplastic carcinoma of the uterus (3). However, uterine carcinosarcomas will be described in this chapter. A key concept of uterine sarcomas is that they are truly heterogeneous tumors with vastly different clinical presentations, responses to therapy, and outcomes. Much of the available literature, until more recently, is extremely limited by lumping all uterine sarcomas into one cohort.
EPIDEMIOLOGY AND RISK FACTORS
Histologic Distribution
Histopathologic criteria for uterine sarcomas have greatly evolved over the last few years and, therefore, histologic distribution may have shifted. Many series do not include or specify sarcoma subtypes and many are very small in number. Combining series with at least 100 cases including carcinosarcomas, the most common uterine sarcomas are leiomyosarcoma and carcinosarcoma (4–9) (Fig. 23.1A). Leiomyosarcomas account for over two-thirds of uterine sarcomas when excluding carcinosarcomas (4–10) (Fig. 23.1B). Endometrial stromal sarcomas (ESSs) account for approximately 25% of true uterine sarcomas with all of the other subtypes being exceedingly rare and accounting for <10% as a whole (4–10) (Fig. 23.1B). ESSs are by definition now considered all “low-grade.” Undifferentiated uterine sarcoma is now the preferred terminology for what was previously called “high-grade” ESSs. Therefore, endometrial stromal sarcoma information in this chapter primarily refers to these “low-grade” lesions. Abeler and colleagues have the most recent and largest series of uterine sarcomas (n = 419) from the Norwegian Cancer Registry, in which histologic subtype distribution was described (10). This series excluded carcinosarcomas altogether. Of these 419 sarcomas, 62% were leiomyosarcomas, 20% were ESSs, and 18% were various other subtypes that included undifferentiated sarcomas (6%), adenosarcomas (5.5%), sarcoma, not otherwise specified (NOS) (4.5%), rhabdomyosarcoma (<1%), giant-cell sarcoma (<1%), and perivascular epithelioid cell tumors (PEComa; <1%) (10).
Age Distribution
Patients with carcinosarcomas and adenosarcomas tend to be older at the time of diagnosis compared to those with leiomyo-sarcomas and ESSs. The mean/median ages reported among published series range from 42 to 51 years for ESSs (4,5,9,10–15), 48 to 57 years for leiomyosarcomas (4,5,9,10,16–18), 57 to 67 years for carcinosarcomas (4,5,9,19,20), 58 to 66 years for adenosarcomas (10,21), and 46 years for undifferentiated sarcomas (11). Subsequently, many patients with carcinosarcomas are postmenopausal at the time of diagnosis, whereas patients with leiomyosarcoma, endometrial stromal sarcoma, or undifferentiated sarcomas may be pre- or perimenopausal at the time of diagnosis. Additionally, the mean age for patients diagnosed with smooth muscle tumors of uncertain malignant potential (STUMP) is 43 years, and many patients are also likely to be premenopausal (22). This would have potential implications in terms of fertility in these patients. The other subtypes are so rare that it is difficult to discern a particular pattern of age distribution.
Racial Distribution
Zelmanowicz et al. (23) noted that women with carcinosarcomas are more likely to be of African American descent (among 453 patients and controls, 28% vs. 4%, p = 0.001) than those with endometrial adenocarcinomas. In the reviews of Mortel et al. and Norris et al. (24,25), 33% and 24% of patients with carcinosarcomas were non-White, respectively. Brooks et al. (26) reported that the age-adjusted incidence of uterine sarcomas in African American women was twice that of controls. The age-adjusted incidences of leiomyosarcomas for Black and White women, respectively, were 1.5 and 0.9 per 100,000. Those for carcinosarcomas in Black and White women, respectively, were 4.3 and 1.7 per 100,000.
Risk Factors
Prior Radiation Exposure
Prior exposure to pelvic radiotherapy is thought to increase the risk of developing a subsequent uterine sarcoma, primarily carcinosarcoma and undifferentiated sarcoma. Prior pelvic radiotherapy is not thought to increase the risk for uterine leiomyosarcoma or STUMP. In a large series from the Mayo Clinic, only 1 (0.6%) of 208 leiomyosarcoma cases had been exposed to prior pelvic radiotherapy (27). None of the 41 STUMP cases described by MD Anderson Cancer Center had a history of prior pelvic radiotherapy (22). Christopherson et al. (28) described two cases among 33 patients with uterine leiomyosarcoma who had a history of radiotherapy.
World Health Organization Classification of Mesenchymal Tumors of the Uterine Corpus |
Mesenchymal Tumors |
Endometrial stromal and related tumours |
Endometrial stromal sarcoma, low grade |
Endometrial stromal nodule |
Undifferentiated endometrial sarcoma |
Smooth muscle tumours |
Leiomyosarcoma |
Epithelioid variant |
Myxoid variant |
Smooth muscle tumour of uncertain malignant potential |
Leiomyoma, not otherwise specified |
Histological variants |
Mitotically active variant |
Cellular variant |
Haemorrhagic cellular variant |
Epithelioid variant |
Myxoid |
Atypical variant |
Lipoleiomyoma variant |
Growth pattern variants |
Diffuse leiomyomatosis |
Dissecting leiomyoma |
Intravenous leiomyomatosis |
Metastasizing leiomyoma |
Miscellaneous mesenchymal tumours |
Mixed endometrial stromal and smooth muscle tumour |
Perivascular epithelioid cell tumour |
Adenomatoid tumour |
Other malignant mesenchymal tumours |
Other benign mesenchymal tumours Mixed Epithelial and Mesenchymal Tumours |
Carcinosarcoma (malignant mullerian mixed tumour; metaplastic carcinoma) |
Adenosarcoma |
Carcinofibroma |
Adenofibroma |
Adenomyoma |
Atypical polypoid variant |
Source: Tumors of the uterine corpus. In: Tavassoli FA, Devilee P, eds. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of the Breast and Female Genital Organs. Lyons, France: IARC Press; 2003:217–258, with permission.
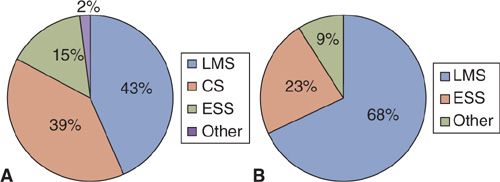
FIGURE 23.1. Histologic distribution of uterine sarcomas in series with at least 100 cases including carcinosarcomas (A) and excluding carcinosarcomas (B).
Source: From Park J, Kim D, Suh D, et al. Prognostic factors and treatment outcomes of patients with uterine sarcoma: analysis of 127 patients at a single institution, 1989–2007. J Cancer Res Clin Oncol. 2008;134:1277–1287; Koivisto-Korander R, Butzow R, Koivisto A, et al. Clinical outcome and prognostic factors in 100 cases of uterine sarcoma: experience in Helsinki University Central Hospital 1990–2001. Gynecol Oncol. 2008;111:74–81; Kahanpaa K, Wahlstrom T, Grohn P, et al. Sarcomas of the uterus: a clinicopathologic study of 119 patients. Obstet Gynecol. 1986;67:417–424; George M, Pejovic MH, Kramar A. Uterine sarcomas: prognostic factors and treatment modalities—study on 209 patients. Gynecol Oncol. 1986;24:58–67; Olah KS, Gee H, Blunt S, et al. Retrospective analysis of 318 cases of uterine sarcoma. Eur J Cancer. 1991;27:1095–1099; Pautier P, Genestie C, Rey A, et al. Analysis of clinicopathologic prognostic factors for 157 uterine sarcomas and evaluation of grading score validated for soft tissue sarcoma. Cancer. 2000;88:1425–1431; Abeler VM, Royne O, Thorensen S, et al. Uterine sarcomas in Norway. A histopathological and prognostic survey of a total population from 1970 to 2000 including 419 patients. Histopathology. 2009;54:355–364, with permission.
ESS, endometrial stromal sarcoma; CS, carcinosarcoma; LMS, leiomyosarcoma; AS, adenosarcoma.
In the series of carcinosarcomas reported by Norris et al. in 1966 (25), 9 of 31 patients (29%) had received pelvic radiotherapy from 7 to 26 years prior to diagnosis. Among 1,208 uterine malignancies in a report by Meredith et al. in 1986 (29), only 30 (2.4%) occurred in patients exposed to prior pelvic radiotherapy. Only 8 of these 30 patients had been irradiated for a gynecologic malignancy; others received pelvic radiation therapy for a benign diagnosis as this was done decades ago. Of irradiated patients, 5 (17%) developed carcinosarcomas, for a crude association of 11%. The risk of endometrial adenocarcinoma arising after radiation was much less at 2%. It has been suggested that postirradiation carcinosarcomas occur at a younger average age than those arising de novo (30). Latency to diagnosis of malignancy is generally shorter in older patients, however (29). Pothuri et al. compared the clinicopathologic characteristics of 23 cases of uterine cancers that occurred in patients with a prior history of cervical cancer treated with pelvic radiotherapy to 527 cases of uterine cancers that developed in patients without a history of pelvic radiotherapy (31). Carcinosarcomas and undifferentiated sarcomas accounted for 9 (39%) of the 23 radiation-associated malignancies compared to only 33 (8%) of the sporadic cases (31). It also seems that these radiation-associated malignancies tend to have a worse outcome, possibly due to lack of early symptomatology. The rarity of the other uterine sarcoma subtypes precludes the ability to make any definitive statements regarding this issue.
Hormone Exposure
There have been some suggestions that exposure to hormonal medications, including tamoxifen, may also increase the risk of uterine sarcomas. The association of estradiol-progestin therapy was recently assessed using data from the Finnish Cancer Registry, which captures nearly 100% of all cancers in Finnland as well as the Reimbursement Registry of the Social Insurance Institution (32). Uterine sarcomas occurred in 76 out of the 243,857 women who were identified as having had used estradiol-progestin therapy for more than 6 months. Carcinosarcomas were not included in this analysis. The ever use of estradiol-progestin therapy was associated with a 60% elevation in the risk for any uterine sarcoma (SIR, 1.6; 95% confidence interval [CI], 1.2–1.9). This association was mostly for leiomyosarcoma (standardized incidence ratio [SIR], 1.8; 95% CI, 1.3–2.4) and not statistically for endometrial stromal sarcoma (SIR, 1.4; 95% CI, 0.9–2.1). Also, this elevated risk was only noted in women who had used estradiol-progestin therapy for 5 years or more. Despite a possible increased relative risk, the overall absolute risk of uterine sarcomas is still exceedingly low, and these data should not dissuade the use of hormones in the appropriate case.
Tamoxifen Use
Tamoxifen use among breast cancer patients may also increase the risk of uterine malignancies, including possibly sarcomas. Lavie and colleagues, using data from 1,507 cases of women with breast cancer in the National Israeli Cancer Registry diagnosed from 1987 to 1988, noted that uterine cancers developed in 17/886 (1.9%) women treated with tamoxifen compared to only 4/621 (0.6%) of those who did not receive tamoxifen (odds ratio [OR], 3.1; 95% CI, 1–9.1) (33). The risk of uterine cancer was associated with increased duration of tamoxifen use. A significant association was seen with more than 4 years of tamoxifen use (OR, 6.6; 95% CI, 2.0–21.1), whereas use of tamoxifen for 2 years or less was not associated with an increased risk of uterine cancer (OR, 2.1; 95% CI, 0.4–11.6). Uterine sarcomas developed in only 4 cases of the entire cohort. These included 2 carcinosarcomas and 2 rhabdomyosarcomas. All 4 occurred in patients who received tamoxifen, suggesting a possible association of tamoxifen use and uterine sarcoma risk. Hoogendoorn and colleagues reported that among patients diagnosed with uterine cancer, carcinosarcomas accounted for a much larger proportion of cases in those who had received prior tamoxifen therapy compared to those who had not (15% vs. 4%, respectively) (34).
Hereditary Predisposition
Hereditary predisposition to certain uterine sarcomas has also been suggested but still remains to be clearly elucidated. Among 164 Hereditary nonpolyposis colorectal cancer (HNPCC) families with disease-predispoing mutations, sarcomas of various sites represented 1% (n = 14) of the 1,570 malignant diagnoses using cases from the Danish HNPCC-register (35). Three of these 14 sarcomas were uterine: one was a carcinosarcoma in a 44-year-old with loss of MSH2 and MSH6 expression in the sarcoma, other was also a carcinosarcoma in a 55-year-old with loss of MSH6 expression, and the still other was a leiomyosarcoma in a 44-year-old with loss of MSH2 and MSH6 expression. Uterine leiomyosarcoma has also been recently suggested to be increased in women with hereditary retinoblastoma (36). The overall risk of uterine sarcoma is still low in these hereditary syndromes but of interest. Prophylactic hysterectomy is strongly recommended in women from HNPCC kindred once childbearing is complete due to the high risk of endometrial carcinoma, but no such recommendation exists for women with hereditary retinoblastoma since this is a recently described potential risk.
CLINICAL PRESENTATION AND DIAGNOSIS
Presenting Symptoms and Signs
The most common presenting symptom of uterine sarcomas is abnormal vaginal bleeding (4,11,13,21,26,36) (Table 23.2). Patients with leiomyosarcomas will also often note an abdominopelvic mass or one that is palpable on physical examination (4,26). A large number of leiomyosarcomas and ESSs are incidentally diagnosed after surgery for presumed uterine fibroids. The presence of endometrial stromal sarcoma was an incidental finding in 42% of cases reported by Memorial Sloan-Kettering Cancer Center (15). Therefore, it is quite likely that the vast majority of cases ultimately diagnosed with leiomyosarcoma or endometrial stromal sarcoma would have had some abnormal enlargement of the uterus discovered after presenting with abnormal vaginal bleeding. Vaginal bleeding in a postmenopausal female should be evaluated appropriately with endometrial sampling and possibly an intravaginal ultrasound. An enlarging pelvic mass in a postmenopausal female should also cause some concern for a malignant process. These symptoms and signs in premenopausal patients are a challenge and the diagnosis of malignancy is missed or often delayed since multiple benign conditions are more likely to be the cause of these symptoms. Carcinosarcoma often presents with vaginal bleeding in a postmenopausal female and often can be diagnosed with endometrial assessments, as it is a lesion that arises in the endometrium (unlike the true uterine sarcoma that often does not have an endometrial component or involve the endometrium).
Common Presenting Symptoms in Patients with Uterine Sarcomas (% of Cases Describing that Symptom) |
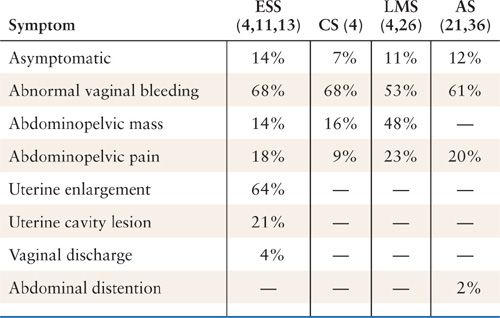
ESS, endometrial stromal sarcoma; CS, carcinosarcoma; LMS, leiomyosarcoma; AS, adenosarcoma.
There are no reliable serum tumor markers for uterine sarcoma. Serum CA-125 is elevated in 17% to 33% of leiomyosarcoma, ESSs, and carcinosarcomas (4). It should not be routinely used in the evaluation and diagnosis of these tumors. Preoperative endometrial assessments with either office pipelle or dilation and curettage (D&C) under anesthesia are limited in the evaluation and correct diagnosis of uterine sarcomas. However, endometrial assessments should be performed in women who present with abnormal vaginal bleeding, and uterine sarcoma may be discovered during this assessment. Bansal et al. reported that an invasive tumor was diagnosed in 86% of uterine sarcoma cases that had undergone a preoperative endometrial sampling (37). The correct histology for uterine sarcoma was only noted in 64% of the cases ultimately diagnosed with uterine sarcoma (37). However, the majority of the sarcomas in the series by Bansal and colleagues were carcinosarcomas on final pathology (32 [70%] of 46 sarcomas), with only 4 leiomyosarcomas, 2 endometrial sarcomas, and 8 other sarcomas diagnosed on final pathology (37).
Preoperative Imaging
Preoperative imaging is limited in differentiating benign from malignant uterine lesions, especially in the absence of obvious extrauterine disease, but it is often available as part of the initial evaluation of these patients. Carcinosarcomas are often diagnosed using endometrial sampling; therefore, preoperative imaging is not important in the diagnosis for carcinosarcomas, but may be valuable in assessing for the presence of extrauterine disease. Small, single-institution, retrospective series have suggested that pelvic MRI may be of value in identifying uterine sarcomas and distinguishing them from benign uterine lesions. Namimto et al. reported a 100% sensitivity and specificity using diffusion-weighted imaging (DWI) and T2-weighted MR imaging in distinguishing between uterine sarcoma and benign lesions compared to DWI alone (38). This seems quite promising but not likely to be possible in clinical practice and not likely to be reproducible reliably. For example, Cornfeld and colleagues reported very poor accuracy for various objective MR imaging criteria in distinguishing leiomyomas with atypical imaging features from malignant uterine mesenchymal tumors (39). Using these various specific MR imaging criteria, the sensitivities for uterine sarcoma ranged from only 17% to 56% but the specificity was 80% to 100% (39).
Serum LDH and MR Imaging
Serum lactate dehydrogenase (LDH) may be an interesting additive to imaging in the evaluation of uterine lesions concerning for leiomyosarcomas (Table 23.3). Goto and colleagues reported an accuracy rate of 99.3% in predicting uterine leiomyosarcoma when combining serum LDH and dynamic MRI in a prospective trial (40). The positive predictive value was 91% using the combined assessment compared to 39% for LDH alone and 71% for MRI (dynamic or not). These results are impressive and would require further validation, but it is simple enough to obtain a serum LDH in patients with concerning lesions of the uterus. Another important point is that the series by Goto and colleagues, as well as other series, note a high specificity and nearly 100% negative predictive value for MRI with or without LDH. This can be useful in women who do not wish to undergo surgery and, especially, in women who are extremely desirous of fertility. It may also help when deciding upon the surgical approach in patients with presumed benign leiomyomas as well as much “concerning” lesions.
Test Characteristics Using Dynamic MRI and Serum Lactate Dehydrogenase (LDH) in Predicting Uterine Leiomyosarcomas from Degenerating Leiomyomas |
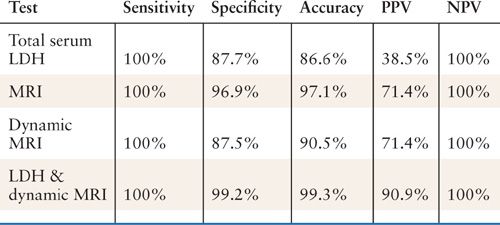
PPV, positive predictive value; NPV, negative predictive value.
Source
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


