D. SCOTT McMEEKIN  CATHERINE YASHAR
CATHERINE YASHAR  SUSANA CAMPOS
SUSANA CAMPOS  RICHARD J. ZAINO
RICHARD J. ZAINO
Endometrial cancer (EC) accounts for nearly 50% of all new gynecologic cancers diagnosed in the United States. It is the fourth most common malignancy in women, and the eighth most common cause of cancer death. The American Cancer Society (ACS) estimated that there were 47,130 new cases of endometrial carcinoma and 8,010 deaths from advanced or recurrent disease in 2012 (1). The death rate per 100,000 women from all malignancies in the United States was 160.49, with uterine cancers contributing at a rate of 4.13 (1). Worldwide, EC is only second to cervical cancer in frequency. The ACS reported that the incidence of EC rose 13% from 1987 to 2012; however, the numbers of deaths rose ~250% in the same time period. Endometrial carcinoma occurs most often in the sixth and seventh decades of life, with an average age at onset of 60 years. It is estimated that 75% to 85% of the cases occur in patients 50 years old and older, and 95% occur in patients over 40 years of age (2, 3). The disease, although reported in patients as young as age 16 years, is rare in patients younger than 30 years of age.
EC is commonly confined to the uterus at diagnosis. Data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program demonstrated that Stage I disease was found in 73% of patients, and 10% had Stage II disease (4). The 26th Annual Report of the International Federation of Gynecology and Obstetrics (FIGO) on 9,386 EC patients demonstrated that 83% of patients were Stage I or II (5). With the favorable disease distribution at presentation, it is not surprising that most patients have a favorable prognosis. Results from FIGO show that 85% to 91% of Stage I patients are alive at 5 years, and patients in the SEER database with localized disease have 96% 5-year survival (4, 5). As a result, EC has been considered a “good cancer”; that is, most patients present with early-stage, highly curable disease. Despite the favorable characteristics for most patients, those with high-risk factors, including increased age, higher tumor grade, aggressive histology, and advanced stage, face real challenges.
The management of EC has undergone continued evolution. In the past, most patients received some form of pre- or postoperative radiation in combination with a simple hysterectomy. With a better understanding of the relationship between uterine factor and risk of nodal disease and recurrence, selective use of surgical staging was integrated. This was followed by an era where, increasingly, surgical therapy expanded to include routine use of pelvic and paraaortic lymphadenectomy. Minimally invasive techniques were studied and have been routinely adopted into the management of women with EC. Today, greater emphasis is being placed on the selection of particular patients for whom lymphadenectomy may best offer better outcomes, and whose avoidance may result in less morbidity. Understanding tumor biology as it relates to predicting recurrence and survival, and how genetic changes can be exploited to direct postoperative therapies, represent our current challenge. Over 10 years ago, the National Cancer Institute convened an expert panel to develop a national 5-year plan for research priorities in gynecologic cancers. The resulting report, Priorities of the Gynecologic Cancer Progress Review Group (PRG), specified that understanding tumor biology was the central key toward controlling gynecologic cancers. For EC, one of the top research priorities defined by the PRG was to identify prognostic and predictive markers for treatment efficacy and toxicity. In a 2006 State of the Science Meeting, in Manchester United Kingdom, a series of research questions on prevention, adjuvant treatment, and treatment of advanced or recurrent disease were proposed setting the stage for a clinical trials agenda over the next 5 years (6). In 2011, the Society of Gynecologic Oncologists conveyed a panel of experts that produced a report “Pathway to Progress in Women’s Cancers”. That report detailed areas of research, by disease site, on which the women’s cancer community should focus for the next decade. For EC, the suggested road map for the future of women’s cancer care research would include research related to obesity, predicting risk of metastatic disease, targeting therapy based on risk factors and molecular characteristics of disease, and cost-effective care to suggest prioritization of research strategies (7).
Current clinical controversies center on identifying which patient populations might benefit most from lymphadenectomy, developing alternatives to performing lymphadenectomy, and creating risk models to assist with selection of patients who may benefit most from adjuvant therapies. While we suspect that an increased use of surgical staging has translated to a better understanding of risk, data also suggest that despite negative nodes, uterine factors play a significant contribution to risk of recurrence. Current trends also suggest a less frequent use of pelvic radiation therapy or no use of any radiation (8). There have been important developments in chemotherapy in EC. Combination chemotherapy is increasingly used in the primary management of advanced and recurrent disease, and may hold promise in an adjuvant setting. How to best integrate radiation therapy and which specific techniques to consider are being studied in going clinical trials. Hormonal therapy remains an important option and our understanding of steroid receptors at a molecular level may help to determine which patients may benefit most (9). Enhanced understanding of biologically relevant targets has fostered the development of new classes of agents that attempt to exploit susceptible pathways in tumor cells, and targeted agents are being integrated into large clinical trials (10, 11).
ANATOMY
The uterus is a fibromuscular pelvic organ situated between the bladder and the rectum, and enveloped by peritoneal reflections. It is divided into the fundus, isthmus, and cervix. The uterine wall is composed of the outer smooth muscle myometrium and inner cavity lined by glandular endometrial epithelium with supporting stroma (endometrium). Five paired ligaments cover or support the uterus: broad, round, utero-sacral, cardinal, and vesico uterine. The utero-sacral and cardinal ligaments provide the greatest support within the pelvis and, contrary to cervical cancer, are infrequently involved with tumor spread. Blood is supplied to the uterus by the uterine artery, a branch of the hypogastric artery, which enters the wall of the uterus at the isthmus after it crosses over the ureter. It anastomoses with the ovarian artery in the ovarian ligament.
Malignant transformation of the endometrium is manifest in many fashions based on the anatomical relationships. Tumor growth may be confined to the endometrium, invade the underlying myometrium, penetrate to the uterine serosal surface or adjacent bladder or rectum, or extend into the cervical canal and invade cervical glands or stroma. Peritoneal disease spread may occur via transmigration from the fallopian tubes or through serosal penetration. Hematogenous spread is not uncommon in EC.
The lymphatics of the myometrium drain into the subserosal network of lymphatics, which coalesce into larger channels before leaving the uterus. Lymph flows from the fundus toward the adnexa and infundibulopelvic ligaments. The lymph flow from the lower and middle thirds of the uterus tends to spread in the base of the broad ligaments toward the lateral pelvic sidewall. There are four drainage channels from the uterus: from the fundus; in the folds of the broad ligament, along the mesosalpinx and fallopian tubes; and along the round ligaments. The drainage sites are principally reflected in metastatic potential to pelvic and paraaortic lymph nodes (LN), and occasionally involve inguinal nodes.
EPIDEMIOLOGY AND RISK FACTORS
The most important risk factor for the development of EC is age. EC is primarily a disease of postmenopausal woman, with median age at cancer diagnosis of 60 years (5). Approximately 85% of cases occur after the age of 50, the peak age-specific incidence is from 75 to 79 years (109 per 100,000), and only 5% of cases are from patients younger than 40 years of age (2, 3, 12). The ACS showed the probability of developing a uterine cancer to be one in 142 from age 40 to 59, one in 124 from age 60 to 69, and one in 78 from age 70 and older (1). Not unexpectedly, with an aging U.S. population, the total number of cases of EC has shown yearly increases, whereas the annual age-adjusted incidence rate peaked in the mid-1970s (33.8 per 100,000) and has remained stable at 23 to 25 cases per 100,000 women over the last 10 years (1).
Race also appears to play a role in the development of EC. The rates of EC are highest in North America and northern Europe, lower in eastern Europe and Latin America, and lowest in Asia and Africa (13). Factors accounting for these findings include differences in rates of obesity, use of hormone replacement therapy (HRT), and reproductive factors. In the United States, non-Hispanic White women have the highest age-adjusted incidence of EC at 25.4 (per 100,000 women), compared to women of African American (19.5), Asian (15.8), or Hispanic (17) heritage (14). African American women, however, have a much higher mortality rate (7.1 vs. 3.9 per 100,000) and lower 5-year survival (61% vs. 84%) compared to non-Hispanic White women. Relative survival in White women exceeds that for African Americans by at least 7% at every stage of diagnosis (1). In the 2012 ACS report, incidence rates of uterine caners in White women were noted to have stabilized, but have increased by nearly 2% per year for African American women since 2004 (1). Multiple explanations have been suggested to explain the differences in outcomes between racial groups, including differences in frequency of high-risk tumor types, differences in access to care (reduced use of surgery and radiation), and differences in medical comorbidities among races. Data from SEER demonstrated that African American women were more frequently diagnosed with higher stage, grade, and high-risk histologies than non-Hispanic White women, but there was no difference in the frequency of recommended therapy types between races (4). In two analyses of nearly 1,200 patients with advanced or recurrent EC participating in phase 3 chemotherapy trials conducted by the Gynecologic Oncology Group (GOG), African American race was independently associated with a lower likelihood of response to chemotherapy (relative odds of response 0.62) and decreased overall survival (OS) (hazard ratio [HR], 1.26) compared to White women (15, 16). These results suggest that racial disparity in outcomes exist even though patients were treated in similar fashion. Interestingly, one small study comparing microarray-based expression profiling between stage-, grade-, and histology-matched African American and Caucasian patients found no clear differences in global gene expression profiles, suggesting that environmental or social issues played a greater role in explaining disparity (17).
Most cases of endometrial carcinoma are thought to be sporadic; however, some cases clearly have a hereditary basis. The Lynch syndrome (hereditary nonpolyposis colorectal cancer [HNPCC]) is an autosomal-dominant cancer susceptibility syndrome associated with early-onset colon, rectal, ovary, small bowel, ureter/renal pelvis cancers, and EC. Lynch syndrome–related ECs account for 2% to 5% of all ECs, and occur in nearly 10% of women diagnosed with EC less than 50 years of age (18). The lifetime risk of EC in Lynch syndrome women is 40% to 60%, a risk similar to that of developing colon cancer. The risk of ovarian cancer is 10% to 12%. In about 50% of cases where patients have both colonic and gynecologic cancers (endometrial or ovarian), the gynecologic cancer precedes the diagnosis of colon cancer (19). The syndrome is most commonly due to germ-line mutations of one of the DNA mismatch repair genes MSH2, MLH1, or MSH6. In one study, 23% of EC patients diagnosed at less than 50 years of age with one relative having a Lynch type cancer had a mismatch repair gene mutation (20). Prophylactic hysterectomy and bilateral salpingo-oophorectomy has been shown to be an effective strategy for preventing ovarian and ECs in these high-risk patients (21).
Controversy exists regarding the relationship between BRCA1 and BRCA2 mutations and the risk of EC. Germ-line mutations of BRCA1 and BRCA2 account for a large proportion of hereditary breast and ovarian/primary peritoneal cancers. In 1999, Hornreich et al. presented a case report of sisters with the same BRCA1 mutation who were diagnosed with serous carcinomas of the uterus and suggested a possible association (22). Several larger studies have attempted to address this hypothesis. In one study of 199 Ashkenazi Jewish patients with EC from a single institution, Levine et al. genotyped all patients for founder BRCA1 and BRCA2 mutations that existed in that patient population. The frequency of germ-line mutations (3 per 199, 1.5%) in EC patients was comparable to the baseline rate of 2% in the Ashkenazi population, suggesting no increased risk (23). In a large prospective study by Beiner et al., 857 known BRCA1 and BRCA2 carriers aged 45 to 70 were followed over time for the development of EC (24). With an average length of follow-up of 3.3 years, six women developed EC. Four of the six patients had used tamoxifen. Compared to the expected rate of EC in a general population, BRCA carriers who did not receive tamoxifen did not have a significant increase in risk of developing EC, whereas those patients who had received tamoxifen had a 11.6 incidence ratio (p = 0.0004). Barak evaluated 289 Jewish women with EC (80% type I, 20% type II tumors) for predominant mutations in Jewish populations (BRCA1, BRCA2, MSH2, MSH6 selected mutations). Five women were found with BRCA1/2 mutations, reflecting a rate similar to that seen in the general Ashkenazi Jewish population, and the authors indicated the data did not support screening in based on an EC diagnosis (25).
The clinical picture in endometrial carcinoma is as varied as are the associated risk factors for its development. One of the paradigms for bridging the gaps between epidemiologic, clinical-pathologic, and molecular factors seen in EC types is the relatively simple, yet attractive, classification system of ECs suggested by Bokhman in 1983 (26). ECs are thought to broadly arise from one of two different pathways: estrogen dependent or estrogen independent (Table 22.1). Based on the clinical and histologic features, ECs have been divided into type I and type II tumors. Type I tumors are more common (85%), tend to be found in younger women, and develop via a precursor lesion of atypical hyperplasia. These tumors are associated with a predisposing history of hyperestrogenism. They tend to be well differentiated and have minimal myometrial invasion, and as a result typically have a favorable outcome. Type II tumors account for a small percentage of endometrial carcinomas, occur in an older population, and frequently develop in the face of an atrophic endometrium. About half of all relapses occur in this group. Serous, clear-cell, and perhaps grade 3 tumors fit into the type II category. Despite the broad generalizations of the two categories, translational science data lend support for a separation into these groups at a molecular level. For example, mutations of TP53 are common in uterine papillary serous carcinoma (UPSC), and rare in type I tumors (27). In type I tumors, PTEN mutations are common, but are rare with UPSC tumors. Global gene expression profiles have also been shown to be different between type I and II tumors (28).
Endogenous or exogenous exposure to estrogen is believed to be an important risk factor for the development of endometrial hyperplasia and type I cancers (29). Estrogens not opposed by progestins lead to increased mitotic activity of endometrial cells, resulting in more frequent errors in DNA replication and somatic mutations (30). These genetic changes are manifest clinically in endometrial hyperplasia and cancer. Estrogen excess as an etiology for cancers is supported by epidemiologic features of the disease. Patients with chronic anovulation, nulliparity, early age of menarche, and late menopause have classically been identified with EC. Occasionally, endometrial hyperplasia or cancer develops in the setting of an estrogen-producing ovarian tumor (granulosa cell tumor) (31). The use of unopposed estrogens as part of hormone replacement strategies was first defined as an important risk factor in 1975 when the age-adjusted rate for EC peaked at nearly 33.8 per 100,000 (32, 33). A meta-analysis of 30 studies showed that the relative risk of ever users of estrogen therapy was 2.3 compared to nonusers, and it increased to 9.5 in users of 10 or more years (34).
Comparison Between Type I and Type II Endometrial Cancers |
| Type I | Type II |
Clinical Features | ||
Risk factors | Unopposed estrogen | Age |
Race | White > Black | White = Black |
Differentiation | Well differentiated | Poorly differentiated |
Histology | Endometrioid | Nonendometrioid |
Stage | I/II | III/IV |
Prognosis | Favorable | Not favorable |
Molecular Features | ||
Ploidy | Diploid | Aneuploid |
K-ras overexpression | Yes | Yes |
HER2/neu overexpression | No | Yes |
P53 overexpression | No | Yes |
PTEN mutations | Yes | No |
Microsatellite instability | Yes | No |
Obesity is an increasingly common problem in the United States, and is estimated to account for 17% to 46% of EC incidence in postmenopausal women (35). Studies have shown that plasma concentrations of androstenedione and estrogens are correlated with body weight in postmenopausal women (36). Aromatization of androstenedione to estrone in adipose cells is believed to be the principal mechanism of excess estrogen production. While much of the data suggest that the relationship is strongest between estrogen exposure and type I cancers, Weiss et al. demonstrated in a case-control study that the risk of more aggressive tumors (higher grade or higher stage) was also seen with unopposed estrogen therapy, obesity, low parity, and history of diabetes. The authors suggested that the risk of EC was influenced by similar risk factors, regardless of tumor aggressiveness (37). Diabetes has also been associated classically with type I ECs (38, 39). Only non-insulin-dependent diabetes (type 2 diabetes mellitus [DM]), characterized by insulin resistance and elevated insulin levels, appears to be associated with EC (40). Hyperinsulinemia and higher levels of insulin-like growth factor 1 are thought to have neoplastic potential and, coupled with increased estrogen, are responsible for cancer development (41, 42).
Tamoxifen and Endometrial Cancer
Tamoxifen is a selective estrogen receptor modulator (SERM) with antiestrogenic properties in the breast, and estrogenic effects in tissues such as bone, the cardiovascular system, and the uterus. It has been used in prevention and treatment for all stages of breast cancer. An association with tamoxifen and EC was first reported by Killackey et al. in 1985 (43). The strongest data initially implicating tamoxifen use and the subsequent development of EC were published in 1989 by Fornander et al. (44). The investigators reviewed the frequency of new primary cancers as recorded in the Swedish Cancer Registry for a group of 1,846 postmenopausal women with early breast cancer who were included in a randomized trial of adjuvant tamoxifen. They noted a 6.4-fold increase in the relative risk of EC in 931 tamoxifen-treated patients compared to 915 patients in the control group. The dose of tamoxifen in this study was 40 mg/day, and the greatest cumulative risk of developing EC was after 5 years of tamoxifen use.
Fisher et al. published data regarding the association between tamoxifen use and the development of EC when they reported the findings of the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-14 trial (45). Data regarding the rates of endometrial and other cancers were analyzed on 2,843 patients with node-negative, estrogen receptor (ER)–positive, invasive breast cancer randomly assigned to placebo or tamoxifen (20 mg/day) and on 1,220 tamoxifen-treated patients registered in NSABP B-14 subsequent to randomization. The average annual hazard rate for EC in the placebo group was 0.2 out of 1,000 and 1.6 out of 1,000 for the randomized tamoxifen-treated group. The relative risk of an EC occurring in the randomized, tamoxifen-treated group was 7.5. Similar results were seen in the 1,220 registered patients who received tamoxifen. The mean duration of tamoxifen therapy was 35 months, with 36% of the ECs developing within 2 years of therapy. Of the 23 cases of endometrial cancer developing while on tamoxifen, six cases occurred following less than 12 months of exposure, suggesting that some of the cancers may have been present prior to starting tamoxifen therapy.
Any conclusions drawn regarding the risks of tamoxifen treatment in inducing EC must weigh the benefits of tamoxifen in reducing breast cancer recurrence and new contralateral breast cancers. In the B-14 trial, the cumulative rate per 1,000 women with breast cancer relapse was reduced from 227.8 in the placebo group to 123.5 in the randomized tamoxifen-treated group. In addition, the cumulative rate of contralateral breast cancer was reduced from 40.5 to 23.5, respectively, in the two groups. Taking into account the increased cumulative rate of EC, there was a 38% reduction in the 5-year cumulative hazard rate in the tamoxifen-treated group. Thus, the benefit of tamoxifen therapy for breast cancer outweighs the potential increase in EC being reported.
The suspected mechanism for EC development following tamoxifen exposure is thought to be related to its estrogenic effects on the endometrium. As such, type I, low-grade/early-stage cancers would be expected. A report from the Yale Tumor Registry by Magriples et al. suggested that uterine cancers occurring in breast cancer patients on tamoxifen may behave more aggressively and carry a worse prognosis (46). Other studies, however, have not been able to confirm these findings (Table 22.2) (45, 47–49). It would appear from the available literature that there is no difference in the stage, grade, or prognosis of ECs associated with tamoxifen use.
More commonly, breast cancer patients are being managed with different strategies, which may reduce the impact of tamoxifen on EC risk. Next generation SERM agents, such as raloxifiene, are effective in preventing breast cancer and reducing osteoporosis. In a large case-control study of women with (547 cases) and without (1,410 controls) EC, raloxifiene users had a 50% reduction in risk of EC compared to nonusers, and tamoxifen users had three times the odds of developing EC compared to those who had used raloxifiene (50). Compared to SERMs, aromatase inhibitors prevent estrogen synthesis by inhibiting the conversion of androgens to estrogens. Third-generation aromatase inhibitors (anastrozole, letrozole, exemestane) are replacing tamoxifen for many breast cancer patients with ~50% of postmenopausal ER–positive patients receiving aromatase inhibitors (49). In clinical trials, compared to tamoxifen, aromatase inhibitors have lower incidences of vaginal bleeding or EC (50–53). In premenopausal patients, aromatase inhibitors have little activity, and tamoxifen will continue to have a role.
Clinicopathologic Data from Series Reporting on Tamoxifen-Associated Uterine Cancer |
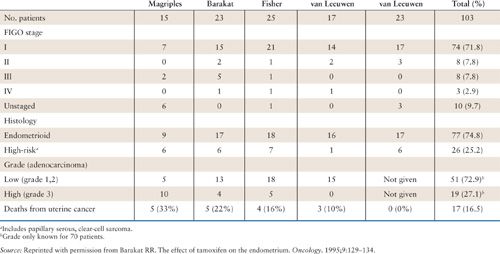
Protective Factors
Factors that reduce circulating estrogen levels (weight loss/exercise, cigarette smoking) appear to be protective against EC. Similarly, progestins antagonize the effects of estrogen on the endometrium and prevent the development of hyperplasia and cancer when added to estrogens (endogenous or exogenous). Combined estrogen–progestin HRT has been associated with reductions in the risk of EC in most, but not all, studies. Prior use of oral contraceptives also appears to be protective against the development of EC (54).
NATURAL HISTORY OF DISEASE
A better understanding of the natural history of EC has developed through evaluation of the patterns of spread. In a landmark study, the GOG performed a surgical pathologic study (GOG 33) in 621 patients with clinical Stage I–occult Stage II EC who underwent a standardized surgical procedure including exploration of the abdomen with biopsy of suspicious findings, collection of peritoneal fluid for cytologic evaluation, abdominal hysterectomy and bilateral salpingo-oophorectomy, and pelvic and paraaortic nodal dissection (55). The results of this study demonstrated important relationships regarding uterine tumor characteristics and spread of disease, and should be ingrained into the memory of those caring for patients with ECs.
Overall, 22% of patients with seemingly uterine confined disease were found to have extrauterine spread. Pelvic and/or paraaortic metastases were found in 11% of patients, 12% had positive peritoneal cytology, 5% had adnexal involvement, and 6% had gross intraperitoneal spread. Nodal metastases were related to tumor grade and depth of myometrial invasion, and patients with positive cytology, or adnexal or intraperitoneal spread also had increased frequency of nodal disease.
Patterns of failure in patients with recurrent EC demonstrate, alone or in combination, hematogenous, lymphatic, intraperitoneal, or local/contiguous spread. As attention has increasingly focused on therapies (surgical, radiation, chemotherapy) to reduce particular sites of recurrences, several have argued for defining relationships between initial disease spread and subsequent risk of recurrence (56). In GOG 33, treatment was not specified by protocol, but results showed that outcomes could be predicted by extent of disease found at surgery, thus demonstrating the important relationship between what is learned at surgical staging and recurrence risk (57).
DIAGNOSTIC EVALUATION
Screening
Many ECs develop by way of a precursor lesion. Estrogen-related cancers frequently develop secondary to atypical endometrial hyperplasia (AEH) or demonstrate AEH in the uterus at the time of hysterectomy. Serous tumors also may develop endometrial intraepithelial carcinoma (EIC) through a precursor lesion (58). Prompt recognition of precursor lesions with institution of proper treatment will prevent cancers and their sequelae. The relatively low prevalence of EC in the population (5 per 1,000 women >45 years) makes standardized screening inefficient. There are a few uncontrolled studies lending some support to the efficacy of screening programs (59, 60). No randomized trials have been published. No health economic data have been presented in relation to the published reports. The American College of Obstetrics and Gynecology (ACOG) and the Society of Gynecologic Oncology do not recommend routine screening of patients for uterine cancer (61, 62). The ACS does recommend annual endometrial biopsies starting at age 35 for women known to have or be at risk for HNPCC.
In lieu of routine screening, prompt assessment of symptomatic patients and those at high risk should be considered. Because 95% of endometrial carcinomas occur in women of 40 years old and older, and because endometrial hyperplasia tends to occur in premenopausal and perimenopausal women, it is appropriate to evaluate individuals past their fourth decade of life if there is abnormal bleeding. Similarly, a higher degree of suspicion should be held for younger patients with high-risk characteristics including significant obesity, polycystic ovarian syndrome/chronic anovulation, or tamoxifen exposure.
Prevention
Due to the increased risk of EC associated with unopposed estrogens, women with an intact uterus should rarely, if ever, be prescribed estrogen-only replacement therapy. The addition of progestins to the regimens of patients treated with exogenous estrogen may prevent endometrial hyperplasia and protect against the development of carcinoma (54). Continuous or sequential progestin regimens may be used, but the most important factor is administration of a progestin for at least 10 to 14 days each month. In patients with chronic endogenous estrogen exposure such as obese women with polycystic ovarian syndrome or chronic anovulation, and perimenopausal women with menometrorrhagia, periodic treatment with a progestin to create scheduled withdrawal bleeding and prevent hyperplasia may be considered (63).
In most cases, patients with hyperplasia with atypia should be treated by vaginal or abdominal hysterectomy to prevent the development of EC (64). Surgery is the definitive therapy as it stops bleeding, prevents cancer, and alleviates the potential of medical failure. Patients with AEH remain at high risk for recurrence of AEH or cancer during their lifetime, even after successful medical therapy (65). Most importantly, despite a preoperative diagnosis of AEH, many patients will be found to have a cancer at the time of hysterectomy (65, 66). Older data suggested that if the endometrial sample is obtained by a biopsy or curettage, 15% to 25% of patients with the diagnosis of atypical hyperplasia may have a uterine carcinoma (67). Prospective data from a large surgical-pathologic trial conducted by the GOG demonstrated that, of 289 patients with a community diagnosis of AEH, 40% had an EC (66). Neither the type of preoperative endometrial biopsy (office endometrial biopsy [EMB], or dilatation and curettage [D&C]) nor the use of an expert pathology panel was associated with a better prediction of who had cancer or not. Patients with significant medical comorbidities, advanced age, or those desiring future fertility may be managed with progestational therapy (68). It has been suggested that a D&C should be performed in those patients who will be medically managed with progestins for therapeutic effect (surgical curettage of tissue) and to better define the risk of an unrecognized cancer, while the data to support these practices are limited. When progestins are used to manage AEH, the specific agents, doses, and schedules may mirror those used to manage dysfunctional uterine bleeding, or advanced or recurrent cancer. To assess the success of medical therapy, endometrial biopsies should be performed at 3- to 4-month intervals provided cancer is not identified (69).
Screening and prevention strategies for women on tamoxifen are more challenging. Women with intact uteri who are taking tamoxifen for either treatment or prevention of breast cancer should be informed of the increased relative risk of developing EC with the use of tamoxifen. This risk is balanced by the reductions in recurrence or development of a contralateral breast cancer. Women on tamoxifen should be encouraged to report abnormal bleeding or vaginal discharge. Screening of asymptomatic women on tamoxifen therapy with ultrasound or endometrial biopsies is not recommended (70, 71).
CLINICAL PRESENTATION
The classic symptom of endometrial carcinoma is abnormal uterine bleeding. A variety of conditions give rise to abnormal bleeding, but particular suspicion should be held for postmenopausal women, and women aged 40 and above with high-risk factors. Approximately 10% of symptomatic postmenopausal patients will be found to have a cancer on biopsy (72). In one series, using age >70 years, diabetes, or nulliparity as risk factors, patients with all three factors had an 87% chance of having an AEH/carcinoma diagnosis, whereas only 3% had significant pathology in the absence of all risk factors (73). Additionally, patients with EC may present with vaginal discharge or have a thickened endometrium incidentally noted on ultrasound performed for another reason. Pap smear screening is not designed to identify EC, but occasionally, patients will have abnormal cervical cytology (atypical glandular cells of undetermined significance [AGUS], adenocarcinoma in situ [AIS]). Patients with intraperitoneal disease may present with similar complaints as patients with ovarian cancer such as abdominal distension, pelvic pressure, and pain.
Historically, when EC was a clinically staged disease (FIGO, 1971) fractional D&C was the procedure of choice to evaluate abnormal bleeding. Fractional D&C permitted assessment of the uterine size and allowed for endocervical curettage, important steps in the staging process. The standard procedure starts with curettage of the endocervix prior to cervical dilatation. Careful sounding of the uterus is performed followed by dilatation of the cervix, followed by systematic curetting of the entire endometrial cavity. Cervical and endometrial specimens should be kept separate and forwarded for pathologic interpretation.
Pathologic evaluation of the endometrium provides histologic diagnosis and can identify other etiologies of bleeding such as chronic endometritis, atrophy, polyps, cervical cancer, or unusual histologic variants (carcinosarcoma, serous carcinoma, placental nodule), which may alter management. Tissue evaluation by office EMB or D&C offer similar information when adequately performed. Today EMB has largely replaced D&C as the diagnostic procedure of choice. In the GOG hyperplasia study, 63% of the specimens were from EMB (Vabra, Novak, Pipelle) and 37% were from D&C (66). Results of endometrial biopsies correlate well with endometrial curettings, and the accuracy to detect cancer is 91% to 99% (74, 75). The accuracy of identifying cancers with EMB is higher in postmenopausal patients than in premenopausal, and a positive study has shown cancer is more accurate for identifying disease than it is in excluding it. Cases where office biopsy cannot be obtained (cervical stenosis, patient intolerance of procedure) or results are nondiagnostic should be followed by D&C. In cases of abnormal bleeding that persists despite negative biopsy, additional investigation is warranted.
Hysteroscopy has been advocated as an adjuvant to D&C to improve detection of pathology in the evaluation of postmenopausal bleeding. Whether it improves the sensitivity to detect hyperplasia and cancers is controversial (76). Hysteroscopy is more accurate in postmenopausal patients, and is more accurate in detecting cancer versus other pathology than it is in identifying cancer or hyperplasia versus other pathology. One concern is that hysteroscopy may promote transtubal migration of tumor cells, which can be detected as malignant pelvic washings on cytology. In one retrospective study, an odds ratio of 3.88 for positive cytology was seen in hysteroscopic D&Cs compared to D&C alone, and the authors cautioned against hysteroscopy to evaluate EC (77). Similarly, a review of literature suggested that water-based hysteroscopy was associated with increased frequency of positive cytology at time of hysterectomy (78). Positive peritoneal cytology as the sole extrauterine factor is no longer recognized as a stage-defining characteristic under the FIGO 2009 system, however (79). No prospective studies have been performed to date, and it remains uncertain what effect positive washing produced by hysteroscopy has, if any, on prognosis.
Ultrasound is commonly used as a less invasive tool to evaluate abnormal bleeding. The measurement of endometrial thickness (ET) has been shown to best predict the absence of carcinoma, with a false-negative rate of 4%, using a threshold value of less than 5 mm (80, 81). The specific ET used for a cutoff value depends on the menopausal status of the patient population evaluated and on the use of HRT. For example, postmenopausal patients on HRT have a median ET 2 to 3 mm more than those not on HRT (82). In a meta-analysis of 85 studies, Smith-Bindman et al. reported that a cutoff level of greater than 5 mm would detect 96% of cancers, and would have a 39% false-positive rate (81). Transvaginal ultrasound measuring the lining thickness of the endometrium has excellent negative predictive value for ruling out ECs or hyperplasia when the thickness is less than 5 mm, but provides less information when greater than 5 mm. Given a pretest probability of having EC in a postmenopausal patient with vaginal bleeding of 10%, a normal endometrial stripe is associated with a 1% chance of a cancer. A consensus panel, composed of radiologists, pathologists, and gynecologic oncologists, suggested that when ET is less than 5 mm, the test can be considered negative for EC (83). For patients with ET greater than 5 mm, EMB, D&C with hysteroscopy, or saline infusion sonohysteroscopy should be performed. Saline infusion sonohysteroscopy has been suggested to better define findings in the endometrial cavity noted on ultrasound and provide clearer distinction of polyps, fibroids, and cancers (84). It is more likely to be successful in premenopausal patients than in postmenopausal patients. The role of vaginal ultrasound in the evaluation of bleeding remains somewhat controversial due to the belief of the importance of histology is defining treatment (for benign and malignant conditions) and of the concern for missed cancers. Good clinical judgment would suggest that patients with ET less than 5 mm who have persistent bleeding undergo tissue biopsy.
DIAGNOSTIC WORKUP
Preoperative Assessment
Following the diagnosis of EC, the surgeon must assess the surgical risks of the patient, evaluate the patient for possible metastatic spread, and determine the most appropriate surgical procedure. EC patients are frequently elderly and suffer from obesity, hypertension, diabetes, or cardiac disease. In a series of 595 consecutive patients, Marziale et al. found an operability rate of 87% (85). Preoperative assessment must be performed, occasionally requiring consultation with additional specialists. At a minimum, patients require a thorough examination to evaluate for evidence of cardiac or pulmonary disease, and to determine the surgical approach. A chest x-ray and electrocardiogram (EKG), complete blood count, and assessment of electrolytes and renal function are standard in this population. Preoperative counseling includes obtaining permission to remove the uterus, tubes, and ovaries, and permission for thorough intra-abdominal exploration with biopsy and tumor removal as necessary, including removal of the pelvic and paraaortic LN.
Evaluation of Metastatic Spread
A thorough physical examination may discover suspicious supraclavicular, inguinal, and/or occasional pelvic LN as well as suggest the presence of pleural effusions, ascites, or omental caking. The pelvic examination can suggest cervical, vaginal, or adnexal spread. An assessment of uterine size and mobility is important, particularly in patients being considered for vaginal approaches (laparoscopic-assisted vaginal hysterectomy [LAVH], total vaginal hysterectomy [TVH]). A chest radiograph is done to search for metastatic tumor as well as to evaluate the cardiopulmonary status of the patient. For patients without obvious extrauterine disease, surgery is the next step. In cases where intra-abdominal, gross cervical, or distant disease spread is suspected, additional studies such as CT scans, magnetic resonance imaging (MRI), or cystoscopy and proctoscopy may be needed to assist with surgical planning.
In general, there is very limited need for imaging studies prior to surgery since findings typically do not result in management changes as most patients present with Stage I–II disease, and the surgery is essentially the same for Stage I–III patients. Imaging studies have significant limitations in detecting nodal disease, which tends to be microscopic in 90% of cases (86, 87). In a small series of higher risk patients who underwent preoperative FDG PET/CT, Signrelli and colleagues showed a 78% patient sensitivity and 93% negative predictive value (88). The GOG is conducting an ongoing prospective assessment of PET/CT in patients with endometrial and cervical cancer (GOG 233). In a prospective blinded comparison of the accuracy of preoperative transvaginal ultrasound to frozen section assessment of myometrial invasion, intraopertive frozen section outperformed ultrasound. The sensitivity and specificity of predicting invasion (none, <50%, >50%) of the ultrasound was 75% and 89%, respectively (89). Patient review of systems and clinical examination frequently lead to suspicion of gross extrauterine disease. Imaging studies may be of better use in certain situations. Serous and clear-cell tumors have a greater frequency of extrauterine disease spread, and imaging studies may offer additional information in some cases. In some settings, imaging studies may help some to determine whether to refer to a gynecologic oncologist or perform surgical staging when it would not otherwise be considered. In addition, imaging studies may have their greatest utility in helping to counsel young patients who are considering fertility preservation options. MRI has been suggested to have more value than computed tomography (CT) scans in assessing myometrial invasion, cervical invasion, and nodal disease, with several reports indicating a 75% to 90% accuracy rate for determining muscle involvement (90, 91). However, limitations do exist, making routine use more difficult to recommend (92, 93). At the present time, the only way to accurately diagnose the extent and depth of intrauterine invasion is by histologic examination of the hysterectomy specimen.
Biomarkers which predict the presence of extrauterine disease spread might be useful to triage patients to referral centers, for consideration of surgical staging, or to define risk. A variety of biomarkers have been suggested as possible candidates with CA-125 being the most studied (94, 95). Attempts have been made to correlate CA-125 levels with extent of extrauterine disease as serum levels are frequently elevated in patients with advanced or metastatic EC. This observation was first reported by Niloff et al. in 1984 (96
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


