Algorithm for the management of cord prolapse (RCOG guideline).
Recognition of Cord Prolapse
Cord prolapse should be suspected when there is an abnormal fetal heart rate (FHR) pattern (e.g. bradycardia, decelerations) in the presence of ruptured membranes, particularly if such changes commence soon after membrane rupture. A speculum and/or a digital vaginal examination should be performed when cord prolapse is suspected, regardless of gestation. Mismanagement of abnormal FHR patterns is one of the commonest aspects of substandard care identified in cord prolapse associated with perinatal death [1].
Call for Help
When cord prolapse is diagnosed, urgent help should immediately be called, including (if possible) a senior midwife, additional midwifery staff, the most experienced obstetrician available, an anaesthetist, the theatre team and neonatologist.
If cord prolapse occurs outside hospital an emergency ambulance should be called immediately to transfer the patient to an appropriate obstetric unit. Even if delivery appears imminent a paramedic ambulance should still be called in case of neonatal compromise at birth.
When help arrives, ‘cord prolapse’ should be clearly stated so that all in attendance immediately understand the problem. Staff outside the obstetric unit (midwives, ambulance staff, general practitioners) should liaise directly with the obstetric unit, clearly stating they are transferring in a patient with a cord prolapse, giving an estimated time of arrival at hospital, so that the appropriate hospital staff are aware, and preparations can be made to ensure timely delivery on arrival at hospital.
Prepare for Immediate Delivery and Minimize Cord Compression
As soon as cord prolapse has been recognized, cord compression should be minimized and preparations should be made for immediate, emergency delivery. Unless the patient is fully dilated delivery should be by emergency CS [1].
Reducing Cord Compression
There are several methods described to minimize compression of the cord between the cervix and presenting part.
Digital Elevation
When cord prolapse is diagnosed the presenting part should be digitally elevated away from the cord by maintaining the examination hand within the vagina and applying upwards pressure on the presenting part. Handling of the cord should be kept to a minimum to reduce the risk of cord vasospasm.
Maternal Positioning
The presenting part may be displaced away from the prolapsed cord using different maternal positions: knee–chest position (woman kneeling on bed with her head down and pelvis in the air), or tilting the bed so that the foot of the bed is steeply raised with the mother lying in the left lateral position.
Bladder Filling
If the decision-to-delivery interval is likely to be prolonged, particularly if it involves ambulance transfer, elevation of the presenting part through bladder filling may be helpful [1]. The bladder should be catheterized with an indwelling urinary catheter and filled with 500–700 ml of fluid by connecting a bag of intravenous fluid to the catheter. The catheter should be clamped once 500–750 ml of fluid have been instilled. It is essential to empty the bladder just before attempted delivery, whether this is to be a vaginal delivery or a CS.
When bladder filling was first described there was one neonatal death in 28 cases, with a decision-to-delivery interval of 25–115 min [6]. Two subsequent studies of a total of 112 cases of cord prolapse managed with bladder filling reported no fetal deaths despite an average decision-to-delivery interval of over 30 min [1].
Assess Fetal Well-being
The fetus should be continuously monitored once cord prolapse has been diagnosed, if possible. If there is no audible fetal heart an ultrasound scan should be performed as soon as possible to confirm fetal viability.
Prepare for Immediate Delivery
Wide-bore intravenous access should be established and blood taken for full blood count and group and save. While preparing for immediate delivery, the fetal condition should be optimized. If an oxytocin infusion is running, this should be stopped and a 500 ml bolus of intraveneous fluid given. Tocolysis to inhibit uterine contractions can be considered (e.g. terbutaline sulphate 0.25 mg subcutaneously). However, although terbutaline has been demonstrated to reduce contractions and abolish bradycardia in the absence of cord prolapse, there is no direct evidence that terbutaline is of benefit during cord prolapse [1]. Manipulation of the cord or exposure to air may cause reactive vasoconstriction and fetal hypoxia/acidosis, therefore some authorities advise that swabs soaked in warm saline wrapped around the cord may be beneficial, but there are no data to support or refute this [1].
Although the measures described above may be useful during preparation for delivery, delivery must never be delayed.
Delivery
Emergency CS is the recommended mode of delivery when vaginal delivery is not imminent. Caesarean section is associated with lower perinatal mortality and low Apgar score at five minutes compared to spontaneous vaginal birth. However, when vaginal delivery is imminent, outcomes appear to be similar or better with vaginal delivery.
There is poor correlation between the decision-to-delivery interval and umbilical cord pH [1]. Neonatal outcomes after emergency CS occurring up to 60 minutes from decision appear to be no worse than those following immediate delivery, in cases without a non-resolving bradycardia. However, a CS should be performed within 30 minutes (category 1) if there are fetal heart rate abnormalities associated with cord prolapse. A CS of urgency category 2 is appropriate in cases where the fetal heart rate is reassuring.
In the majority of cases regional, rather than general, anaesthesia may be used; however, prolonged and repeated attempts at regional anaesthesia must be avoided. The presenting part should be kept elevated while the anaesthesia is undertaken. Clear communication about the urgency and timing of delivery is required between the obstetric and anaesthetic team to ensure the safest method of anaesthesia for both mother and fetus.
Vaginal birth can be attempted at full dilatation when it can be rapidly accomplished, ideally within 10 min of the diagnosis. Ventouse or forceps delivery should only be considered if the prerequisites for operative delivery are met. In general, poor fetal outcomes are associated with more difficult attempts at achieving vaginal birth. In multiparas, or for second twins, a ventouse extraction may be attempted by experienced operators at 9 cm dilatation when there is cord prolapse with severe CTG abnormalities and delivery is considered easily achievable. Breech extraction may be performed under some circumstances (e.g. after internal podalic version for the second twin or when delivery is imminent in singleton breech presentation).
Neonatal Resuscitation
An experienced neonatal team must be present at delivery to ensure full cardiorespiratory support is given, if required, to the neonate.
Documentation
Documentation should include the time cord prolapse occurred, the time help was called and arrived, methods used to alleviate cord compression, the time the decision to deliver was made, as well as the time and mode of birth. The neonatal condition at birth should also be documented. This should include the Apgar score at 1, 5 and 10 min of age and arterial and venous cord pH levels.
Shoulder Dystocia
Definition and Incidence
Shoulder dystocia is a vaginal cephalic delivery that requires additional obstetric manoeuvres to deliver the fetus after gentle downward traction has failed [7]. Shoulder dystocia occurs when either the anterior, or less commonly the posterior, fetal shoulder impact on the maternal symphysis or the sacral promontory respectively, preventing delivery of the body after delivery of the fetal head. The incidence of shoulder dystocia in the largest case series (34 800–267 228 births) ranges between 0.58% and 0.70% [7].
Shoulder dystocia remains a largely unpredictable event that can result in serious long-term morbidity for both mother and baby. Brachial plexus injury (BPI) is the most frequent serious neonatal morbidity associated with shoulder dystocia. The incidence varies substantially between studies [7,8] (2–16% of births complicated by shoulder dystocia), suggesting that at least some of these injuries may be preventable. Aside from any personal harm and health costs, poor outcomes can result in very significant litigation costs. In England, the NHS Litigation Authority paid more than £100 million in legal compensation over a decade for preventable harm associated with shoulder dystocia [9], and in the United States shoulder dystocia is the second most commonly litigated complication of childbirth [10].
Pathophysiology of Shoulder Dystocia
In the majority of women the antero-posterior diameter of the pelvic inlet is narrower than the oblique or transverse diameter. Shoulder dystocia occurs when the diameter of the maternal pelvis through which the fetal shoulders attempt to pass is less than the bisacromial diameter of the fetus, usually when the fetal shoulders do not rotate to the wider oblique pelvic diameter.
Antenatal Risk Factors for Shoulder Dystocia
Macrosomia
The greater the fetal birth weight, the higher the risk of shoulder dystocia [11]. A review of vaginal births of infants born to non-diabetic mothers reported rates of shoulder dystocia of 5.2%, 9.1%, 14.3% and 29.0% in infants weighing 4000–4250 g, 4250–4500 g, 4500–4750 g and 4750–5000 g respectively [12]. Infants weighing over 4000 g are significantly more likely to suffer shoulder dystocia compared to those weighing less than 4000 g (11.1% and 0.6% respectively) [13].
Previous Shoulder Dystocia
Previous shoulder dystocia is a risk factor for recurrent shoulder dystocia. The recurrence rate is reported to be approximately 15% [7]. However, rates may be underestimated due to selection bias; elective CS may be performed in some pregnancies following shoulder dystocia.
Maternal Diabetes Mellitus
Maternal diabetes mellitus increases the risk of shoulder dystocia [8]. Infants of diabetic mothers have a three- to four-fold increased risk of shoulder dystocia compared to infants of non-diabetic mothers for the same birth weight.
Instrumental Delivery
Compared to a spontaneous delivery, shoulder dystocia is approximately twice as likely to occur with instrumental delivery [12].
Maternal Obesity
Shoulder dystocia is associated with obesity; however, obese women tend to have larger babies and the association may be due to fetal macrosomia rather than maternal obesity per se. In a study that controlled for potential confounding effects of other variables associated with obesity, there was no significant increase in the risk of shoulder dystocia associated with maternal obesity (OR 0.9 [95% CI 0.5–1.6]) [14].
Gestational Age
Studies investigating births between 37 and 43 weeks’ gestation suggest there is no significant difference in the gestational age at delivery of births complicated by a shoulder dystocia and births without shoulder dystocia.
Prediction
Macrosomia alone is a weak predictor of shoulder dystocia. The majority of infants with a birth weight of ≥4500 g do not develop shoulder dystocia [15] and, equally importantly, 30–48% of shoulder dystocia occurs in infants with a birth weight of less than 4000 g. Furthermore, antenatal detection of macrosomia is poor. Clinical fetal weight estimation is unreliable; third trimester ultrasound scans have at least a 10% margin for error for actual birth weight and sensitivity of just 60% for macrosomia (>4.5 kg).
A retrospective review of 267 228 vaginal births reported that even the most powerful predictors for shoulder dystocia have a sensitivity of just 12% and positive predictive value of under 5% [16]. The majority of cases of shoulder dystocia occur with women with no risk factors. Shoulder dystocia is, therefore, an unpredictable and largely unpreventable event. Clinicians should be aware of existing risk factors but must always be alert to the possibility of shoulder dystocia with any delivery [7].
Prevention
Shoulder dystocia can only be prevented by CS. However, a decision-analysis model estimated that an additional 2345 caesarean deliveries would be required to prevent one permanent injury from shoulder dystocia [17]. Estimation of fetal weight is unreliable, and the large majority of macrosomic infants do not experience shoulder dystocia; therefore elective CS is not recommended in cases of suspected fetal macrosomia [7]. However, elective CS should be considered for a woman with diabetes and suspected fetal macrosomia (estimated fetal weight >4500 g), reflecting the higher incidence of BPI in this subgroup, and may be considered if the estimated fetal weight is over 5000 g in non-diabetic pregnancies.
Management
There are numerous techniques described that can be used to relieve shoulder dystocia. The Royal College of Obstetricians and Gynaecology have published an evidence-based algorithm for the management of shoulder dystocia (Figure 12.2) [7].
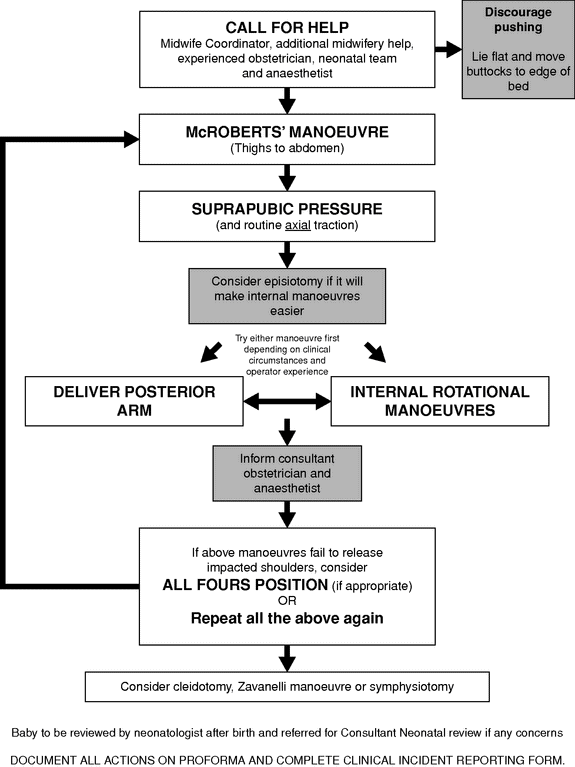
Algorithm for the management of shoulder dystocia (RCOG guideline).
There is no evidence that one manoeuvre is superior to another. The algorithm begins with simple measures, which are often effective, and leads progressively to more invasive manoeuvres.
Recognition of Shoulder Dystocia
There may be difficulty with delivery of the face and chin. When the head delivers it remains tightly applied to the vulva, retracts and depresses the perineum – the ‘turtle-neck’ sign. There may be a failure of restitution and the anterior shoulder then fails to deliver with routine traction.
Call for Help
As soon as shoulder dystocia is suspected, help must be summoned immediately. Help should include (if possible) a senior midwife and additional midwifery staff, the most experienced obstetrician available and a neonatologist. If shoulder dystocia is not resolved quickly then the obstetric consultant and anaesthetist should be urgently called.
Clearly State the Problem
‘Shoulder dystocia’ should be clearly stated as help arrives so that attendants immediately understand the problem. Maternal pushing should be discouraged as it may increase the impaction of the shoulders, and will not resolve the dystocia.
McRoberts’ Position
McRoberts’ position (hyperflexion of the maternal legs) is the most widely advocated first line manoeuvre and was first described in 1983 [18]. McRoberts’ position increases the relative antero-posterior diameter of the pelvic inlet by rotating the maternal pelvis cephaloid and straightening the sacrum relative to the lumbar spine. The reported success rate is between 40% and 90% [8]. McRoberts’ position is associated with less neonatal trauma than other resolution manoeuvres; however, this may be because more severe cases of shoulder dystocia, which are more likely to result in injury, often require more than one resolution manoeuvre.
There is no evidence that using McRoberts’ position in anticipation of shoulder dystocia is helpful; therefore prophylactic McRoberts’ positioning is not recommended [7].
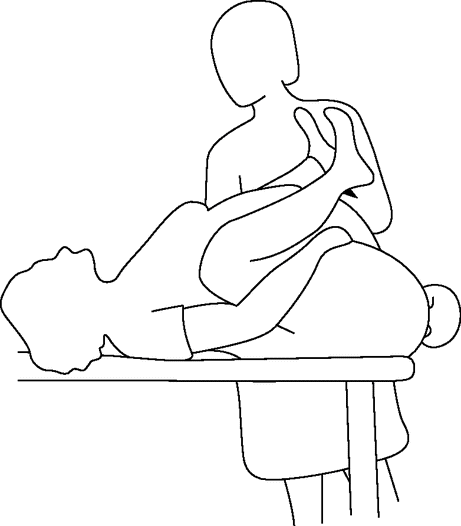
To perform McRoberts’ position, the mother should be laid flat and her legs hyperflexed against her abdomen by an assistant on each side (Figure 12.3). Routine traction (the same degree of traction applied during a normal delivery) should be applied to the fetal head. If the shoulders are not released an additional resolution manoeuvre should be attempted.
The McRoberts’ manoeuvre.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


