Fig. 27.1
Langenskiöld radiographic classification of infantile Blount’s disease. Langenskiöld described six presumably progressive radiographic stages of deformity in infantile Blount’s disease. Stage I is often difficult to distinguish from persistent physiologic varus. Langenskiöld noted that spontaneous correction was possible despite radiographic severity as advanced as Stage IV. Stage VI is characterized by complete medial proximal tibial physeal closure (bar). Reprinted from Langenskiöld A. Tibia vara. Acta Chir Scand 1952; 103:9, with permission from John Wiley & Sons
Adolescent Blount’s disease (Fig. 27.2a) is characterized by later onset, with more subtle physeal and much less epiphyseal distortion. Interestingly, there are neither radiographic classifications nor descriptions of the natural history of adolescent Blount’s disease during remaining growth: steady progression of varus deformity once onset during the remainder of growth is assumed.
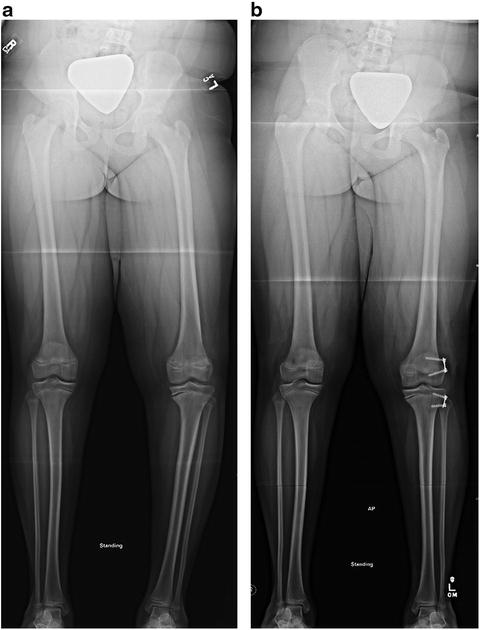

Fig. 27.2
Radiographic appearance of adolescent Blount’s disease. (a) The characteristic radiographic features of Adolescent Blount Disease include varus deformity of the proximal tibia and medial proximal tibial physeal widening. Distal femoral varus deformity (accentuating the varus deformity) and presumably secondary distal tibial valgus deformity may be present. (b) Radiographic appearance after growth modulation of lateral distal femur, lateral proximal tibia, and proximal fibular epiphysiodesis. Note the residual leg length inequality
There is some controversy as to the existence of an intermediate form of Blount’s disease (called “juvenile” by Thompson) [4, 5]. This form is characterized as intermediate in age of onset and radiographic severity of epiphyseal and physeal distortion. At best, this form is much less common that either infantile or adolescent Blount’s disease. Some cases have been described as having an appearance of a “slipped proximal tibial epiphysis” with diffuse widening and irregularity of the physis, and the impression of medial displacement of a relatively intact epiphysis to produce the varus deformity [5] (Fig. 27.3).
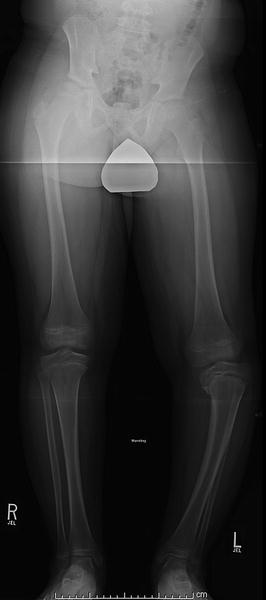

Fig. 27.3
Nine-year-old boy presenting with progressive varus deformity of the left tibia. Note the “intermediate” nature of the patient by presenting age and radiographic epiphyseal/metaphyseal distortion (between “infantile” and “adolescent” Blount’s disease). Note the irregularity of the entire proximal tibial physis. Some authors refer to such intermediate cases as “juvenile Blount’s disease”
Differential Diagnosis
The most difficult differential diagnosis for infantile Blount’s disease is the distinction between persistent physiologic varus and early infantile Blount’s disease. Some authors report the efficacy of the metaphyseal-diaphyseal angle in providing this distinction [6–9]. The problem is compounded by the opportunity for true infantile Blount’s disease to resolve spontaneously (as reported originally by Langenskiöld [3], and for presumably true persistent physiologic varus to progress to infantile Blount’s disease.
Box 27.1. Differential Diagnosis of (Progressive) Varus Deformity in Children
Persistent physiologic varus
True infantile Blount’s disease (resolving or progressive)
Renal-metabolic disorders (vitamin D deficiency, renal osteodystrophy)
Vitamin D-resistant rickets (VDRR)
Thromocytopenia absent radius (TAR) syndrome
Focal fibrocartilaginous dysplasia of the proximal tibia
Epiphyseal dysplasias (multiple; spondylo-epiphyseal; metaphyseal dysostosis)
Other disorders to consider are epiphyseal dysplasias, dwarfing syndromes, thrombocytopenia-absent-radius (TAR) syndrome, and metabolic bone disease (true vitamin D deficiency, vitamin D resistant (hypophosphatemic) rickets, and the mimicking metaphyseal dysostoses (Schmidt and Jansen types) (Figs. 27.4 and 27.5).
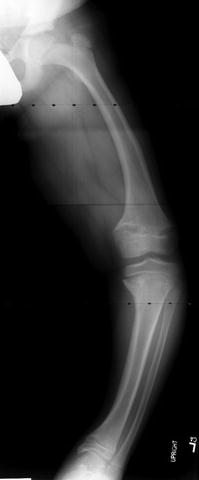
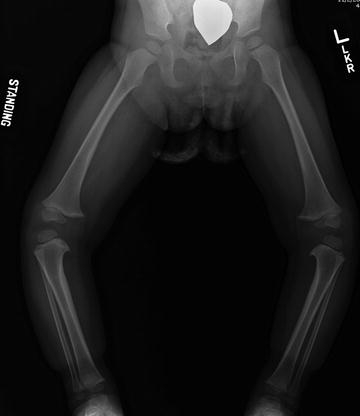

Fig. 27.4
Anteroposterior radiograph of the left lower extremity of a 13-year-old male with poorly controlled vitamin D-resistent rickets (VDRR). Note significant varus deformity of the distal femur, proximal tibia, and distal tibial as well as widening and irregularity of all physes

Fig. 27.5
Anteroposterior radiograph of a 3-year-old girl with thrombocytopenia-absent-radius (TAR) syndrome. Note the apparent medial proximal tibial epiphyseal defect and severe resulting varus deformity of the legs. The patient has no thumb or radius
Natural History
Langenskiöld reported that spontaneous correction can occur in infantile Blount’s disease, even occasionally in advanced stages [3]. While this certainly seems to be true in milder stages, our experience has been less fortunate in later stages. Thus, the short-term natural history for infantile Blount’s disease may be to progress or resolve. The long-term natural history is towards premature intra-articular pathology or early degenerative joint disease, even when angular deformity has been addressed, presumably because of the associated epiphyseal distortion [10, 11].
Interestingly, the short-term natural history of adolescent Blount’s disease is not known: no documentation of radiographic changes during growth has been reported. In general, patients appear to have a more benign long-term natural history with respect to degenerative arthritis [10, 12]. However, the association of adolescent Blount’s disease with morbid obesity, and the recent reports of the failure of deformity correction to impact body mass index portend poorly for the general health and longevity of patients with associated obesity [13–18].
Treatment
Controversies in the Treatment of Infantile Blount’s Disease
Does Bracing Work?
There are several studies that address the question of efficacy of bracing in the prevention of progression, or more importantly, the resolution of deformity in infantile Blount’s disease [19–21]. Unfortunately, these studies are retrospective, and lack the ability to address fundamental questions such as patient compliance with treatment protocols. Furthermore, wear protocols (day, night, or day-and-night) also vary among published studies. It is not surprising then that authors have arrived at different conclusions, that is, “bracing is effective” (in specific cases) or “bracing is ineffective”. We still typically prescribe long-leg, anti-varus braces in patients who are age three or less, and have radiographic Langenskiöld stage II or less infantile Blount’s disease, based on our intra-institutional anecdotal and retrospective experience. We further are skeptical about the efficacy of bracing in patients with bilateral infantile Blount’s disease, although we may prescribe them even in such cases from time to time, if the clinical picture (e.g., family expectations, resistance to surgery, or similar scenarios) seems to warrant a trial of bracing.
Growth Modulation
Whether identifiable in the published literature or not, it is likely that any experienced pediatric surgeon approaches high tibial osteotomy with trepidation, based on some previous bitter or frightening experience with the major acute complications of that surgery, specifically, compartment syndrome, deep infection, or peroneal nerve injury (never mind skin irritation, challenging fixation/casting scenarios, and recurrent deformity). In that context, the wise surgeon always seeks a more benign treatment, and will generally recommend a trial of the same, if there is any hope of effectiveness. This is the current state of “growth modulation” in infantile Blount’s disease [22–26]. The concept is tantalizing: implant one of the commercially available 2-hole (or more) plates on the proximal lateral tibia spanning the physis, for the purpose of temporarily tethering growth in that area, thereby avoiding the significant complication risks associated with high tibial and fibular osteotomy, hope to effect gradual correction of deformity with further growth. Peer-reviewed data confirming the efficacy of such treatment is currently limited, but favorable, Scott et al. [24] reporting 89 % success rate in 18 affected limbs. The likelihood of recurrence of deformity after correction and removal of a growth modulation device is not known. More esoteric questions such as lateral tether due to perichondral ring damage by disruption of appositional epiphyseal/physeal growth by the implant in the very young patient or by direct surgical injury; indications for concomitant epiphysiodesis of the relatively overgrown fibula; or management of the often-associated internal tibial torsion and proximal tibial flexion deformities (Fig. 27.6), remain to be clarified in the era of growth modulation. It is clear that such devices, particularly but not exclusively canulated, titanium screws are at risk for fracture [24]. At a minimum, solid, stainless steel screw devices, with or without more robust (i.e., “H” or similar 4-hole plates) are preferred for growth modulation in Blount’s disease. The surgeon and family must contract to regular longitudinal follow up to monitor growth and intervene as needed, and the family should be educated as to the possibility of implant breakage, and failure of this surgical modality, even in the presence of good radiographic indications and apparently technically adequate implant insertion. It is also not clear at what stage of successful correction in the still-growing child a growth modulation plate should be removed, i.e., when the mechanical axis has been corrected, or after allowing the limb to overcorrect into some valgus. Scott et al. [24] reported that three of eight patients with adequate follow up after full correction and implant removal developed mild recurrent varus deformity, although none required treatment. Those results and the adage of overcorrecting varus deformity when high tibial osteotomy is performed in similar patients suggest that the surgeon should allow slight overcorrection to occur before removing the growth modulation plate in skeletally immature patients.
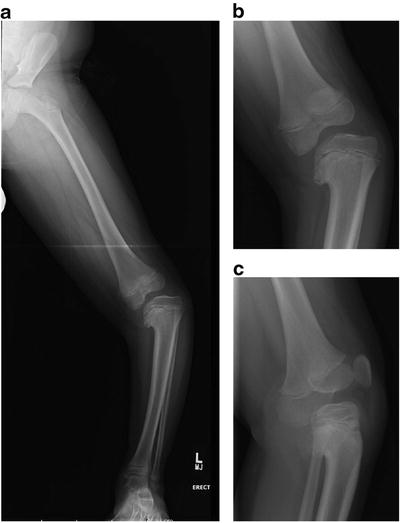

Fig. 27.6
Radiographic appearance of infantile Blount’s disease. (a) Radiographic appearance of left lower extremity. Note the epiphyseal distortion and varus deformity of the proximal tibia, and mild compensatory valgus deformity of the distal femur and distal tibia. (b) Radiographic appearance of the left knee, same patient. (c) Lateral radiograph of the knee. Note the procurvatum deformity of the proximal tibia, and the rotational deformity of the limb
Risks of High Tibial Osteotomy and Acute Deformity Correction
High tibial (below the tibial tubercle) osteotomy of the tibia with concomitant osteotomy of the fibula to “over-correct” the varus deformity is the presumptive “gold standard” management of infantile Blount’s disease [27–30]. Typically, the distal fragment is externally rotated as well (Fig. 27.7a, b). This operation has serious risks, the most important of which are compartment syndrome and direct or indirect injury to the peroneal nerve from manipulation, traction, or entrapment within fascial planes during manipulation of the fragments (Fig. 27.8). Soft tissue irritation, deep infection, and recurrent deformity are also not inconsequential risks. There are several important considerations the surgeon must assess before proceeding:
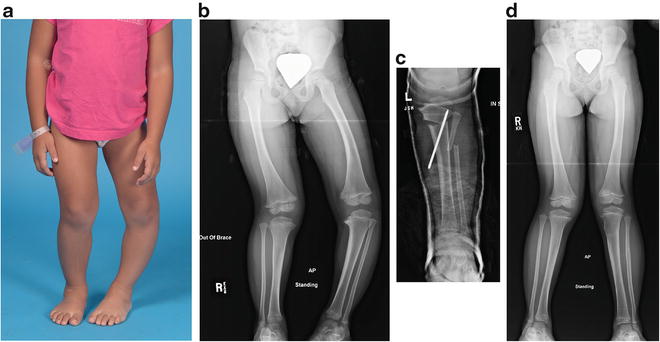
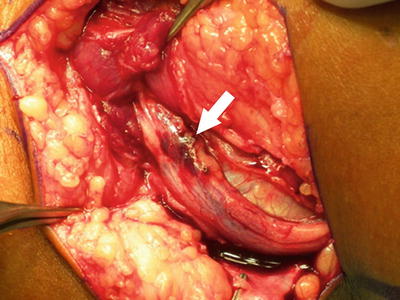

Fig. 27.7
Three-year-old girl with progressive infantile Blount’s disease of the left leg, Langenskiöld stage III, who failed conservative treatment with an anti-varus brace. (a) Preoperative clinical appearance. (b) Preoperative radiographic appearance. (c) Postoperative radiographic appearance. Note the lateral translation of the distal fragment to effect restoration of the tibial mechanical axis. The distal fragment is manipulated to correct both varus and internal rotational deformities associated with infantile Blount’s disease. (d) Radiographic results 2 years postoperatively, age 5 years. Patient has symmetric valgus deformity, with improvement of epiphyseal distortion of the left proximal tibia

Fig. 27.8
Peroneal nerve compression detected and released in the immediate postoperative period. The patient developed peroneal nerve palsy shortly after surgery. Her compartments were soft. At exploration, the nerve was found to be compressed under a fascial band as it passed into the deep anterior compartment. Arrow indicates contused peroneal nerve after release of the fascial band. Same patient as Fig. 27.7
1.
Is the physis still “open” medially, i.e., is the extent of medial physeal involvement less than Langenskiöld stage VI? If the patient has progressed to Langenskiöld stage VI, recurrent deformity is inevitable, and an alternative or adjunct procedure (e.g., completion of epiphysiodesis effect and management of limb length inequality) selected.
2.
What is the current leg length discrepancy, and what’s the plan to manage it?
3.
What are the concomitant deformities, where are they, and how severe are they? Typically, concomitant deformities include distal femoral valgus (variable), proximal tibial “procurvatum” (flexion deformity), and internal tibial torsion. Typically, all of these should be addressed by at least full correction (some argue for “overcorrection” in the skeletally mature patient) to unload the medial proximal tibial epiphyseal load-bearing [30].
The surgeon has to decide on the geometry and type of osteotomy: opening wedge, closing wedge, oblique osteotomies have all be described [27–30]. In contradistinction to adolescent Blount’s, there has been no argument for leaving the fibula intact, presumably because the magnitude of deformity, the desire to overcorrect the varus (coronal plane) deformity, and the need to externally rotate the distal fragment more or less mandate that the fibula be divided to allow full correction. Next to be decided is fixation: cast only, cast and (one or two pins), and external fixation have all been used. Finally, should prophylactic anterior compartment fasciotomy be performed? Some authors support performing a limited anterior compartment fasciotomy in conjunction with tibial osteotomy, while others make no recommendation [27–30]. At a minimum, careful and continuous evaluation of the patient for the presence of compartment syndrome (untoward pain despite adequate release of constrictive splints or casts, or inability to dorsiflex the foot) is essential. In my personal experience, partial prophylactic anterior compartment fasciotomy neither absolutely protects against the development of compartment syndrome, nor is it an entirely innocuous procedure: patients may complain of muscle herniation, discomfort, weakness, or suffer cutaneous nerve injury. The surgeon must carefully weigh his or her strategy in the context of these risks.
Effectiveness of Physeal Arrest Resection
Studies have demonstrated that at least in some cases of infantile Blount’s disease (approximately 50 % in our experience), medial proximal tibial physeal growth may be restored by a variation of Langenskiöld’s physeal arrest resection surgery [31, 32], usually combined with high (below the physis) osteotomy. This procedure is neither universally accepted nor successful. Patients with Blount’s disease typically have an advanced bone age [33]. The wise surgeon will assess the patient’s bone age and the adage of “average” proximal tibial physeal growth of 6 mm per year in the healthy proximal tibial physis to determine if adequate growth remains to warrant an effort at this procedure. A minimum of 4 years of growth remaining would seem to be a reasonable threshold criterion for consideration of physeal bar resection as potentially indicated. Our experience is that even patients who experience resumption of growth will subsequently develop late deceleration/cessation of that growth. Embedding metallic markers or some other method of exact determination of growth persisting is important to allow prompt recognition of secondary cessation of growth (Fig. 27.9a, b). Completion of the epiphysiodesis at that time can save the patient from the development of recurrent deformity requiring an additional osteotomy, and appropriate management of any associated leg length inequality.
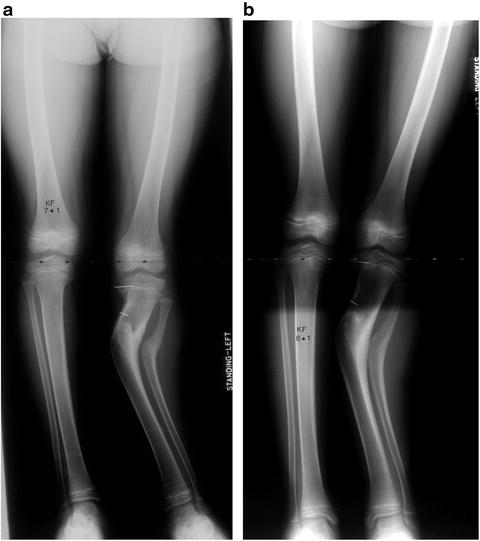

Fig. 27.9
This 6-year, 9-month old girl underwent third-time repeat hight tibial osteotomy, combined with partial physeal bar excision for recurrent infantile Blount’s disease of the left tibia. (a) Four months postoperatively, the patient has persistent iatrogenic valgus deformity at the osteotomy site. Note the metallic markers in the proximal medial tibial epiphysis and metaphysis. (b) Sixteen months postoperatively (1 year after radiograph in part a), the patient has persistent valgus deformity. The markers have separated by approximately 20 mm
Utility of Physeal Arrest Resection Surgery in the Absence of a Bony Physeal Arrest
Andrade and Johnston [34] have recommended “physeal bar resection” surgery in patients with advanced infantile Blount’s disease, but at Langenskiöld stage less than VI, i.e., without demonstrable frank physeal bony bar formation on advanced imaging. They report good (>80 % successful in preventing recurrence of varus deformity, in patients less than 7 years of age) results, but there is not a comparable group treated by high tibial osteotomy alone to confirm the relative efficacy of this procedure.
What to Do with the “Failed” Case?
Usually, the surgeon is faced with many things to consider, when the patient has undergone previous high tibial osteotomy with recurrent deformity. These include: the extent of lower extremity scarring, recurrent (potentially complex) deformity, including variable amount of varus, procurvatum, and internal tibial torsion, leg length inequality in unilateral cases, and potential continued asymmetric growth in the skeletally immature patient (both within the tibia due to the medial arrest, and between the affected and unaffected legs).
There can be no “cookbook” strategy in such cases (Fig. 27.10). The surgeon must individualize the correction plan with careful consideration of all these parameters in the development of a treatment strategy suitable and acceptable to the family and their circumstances. External fixation, with either acute or gradual correction, provides the surgeon with an excellent tool to manage all of these deformities during one treatment stage, if acceptable to the patient and family.
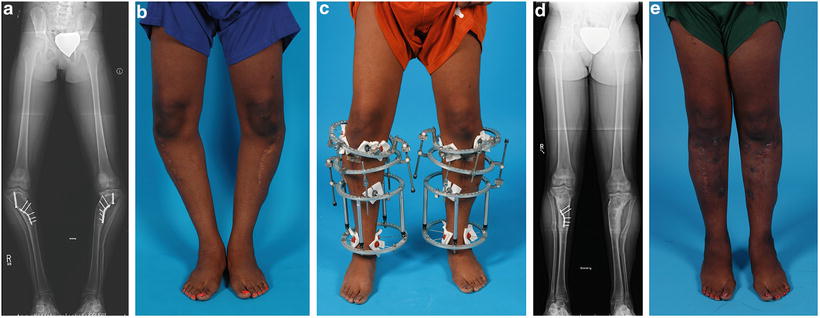

Fig. 27.10
Patient with failed previous treatment of infantile Blount’s disease. (a) Radiographic appearance. The patient has had several prior surgical procedures, including high tibial osteotomies and attempted lateral proximal tibial physeal arrest with retained implants, recurrent varus deformity, and post-surgical proximal metaphyseal valgus deformity. Note the medial translation of the patient’s mechanical axis caused by a failure to lateralize the distal fragment in conjunction with the previous valgus-producing osteotomy. (b) Clinical appearance. (c) Clinical appearance during staged treatment. The patient was managed by staged surgical procedures, including (partial) implant removal, and two-level osteotomies of the tibiae with gradual correction of the varus and valgus deformities using a circular external fixator. (d) Final postoperative radiographic appearance. (e) Final clinical appearance
The surgeon must always keep in mind the fact that patients with infantile Blount’s disease may have premature or “atraumatic” meniscal tears or other degenerative changes, even in the presence of presumably adequate angular and rotational deformity correction [10, 35]. This risk is presumably the result from the epiphyseal distortion that is unique to infantile Blount’s disease and which distinguishes it from adolescent Blount’s disease. Thus, if the patient has significant intra-articular complaints or abnormalities on physical examination, appropriate pursuit of the potential for these structural abnormalities (MRI or arthroscopy) should be carried out.
Is There a Role of “Hemi-Plateau Elevation”?
Radiographs in the advanced stage of infantile Blount’s leave the observer with the distinct impression of a “sagging” or “depressed” medial tibial plateau. This perception (or reality) has led a number of authors to describe “hemi-plateau elevation” [36–38] (in effect, an intra-articular fracture with restoration of the transverse condylar relationships [unless the surgeon is fortunate enough to effect cartilaginous deformation of the articular surface without fracture]). It should be noted that some other authors dispute at least the universal presence of a true depression of the medial plateau, and confirmed the presence of a cartilaginous medial tibial plateau by knee arthrogram [39], or more recently, MRI [35, 40]. The procedure of hemi-plateau elevation has been described in isolation or in conjunction with second osteotomy of the tibia to correct coexisting varus deformity. The surgeon should recognize the most likely event of intra-articular fracture, and the inevitability of no further (favorable) physeal growth, if performing this surgery; thus, completion of the proximal tibial and fibular epiphysiodesis at the time of surgery would seem appropriate. We have not, in our institutional experience, used this procedure to manage even the most severe cases of infantile Blount’s disease.
Box 27.2. Controversies in Infantile Blount’s Disease
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


