KEY POINTS
• Fertility control and family planning should be considered an integral part of health care.
• Contraception should be readily available to any individual or couple that desires it.
• There are a wide variety of effective contraceptive methods available, and most individuals and couples will be able to find a method that fits their needs.
• Long-acting reversible contraception (LARC), including intrauterine devices (IUDs) and contraceptive implants, have seen an encouraging increase in use and hold great promise to reduce the unintended pregnancy rate in the United States and globally.
CONTRACEPTION
Contraceptive Effectiveness
Background
• Table 5-1 presents data on contraceptive effectiveness collected from a variety of sources by Trussell (1).
• The perfect use rates represent the author’s “best guess” of the failure rate during the first 12 months of use among couples who use the method perfectly.
• The typical use rate is the rate of failure in the 1st year of use among average users who may use the method incorrectly or inconsistently.
• In counseling patients about contraceptive choice, it is important to recognize one’s own biases about the methods. It is fairly common for counselors to quote perfect use failure rates for methods that they favor while quoting typical use rates for those they disfavor.
• In the United States, over a third of all women who have used at least one method of contraception have discontinued due to dissatisfaction, but there is a wide variation in discontinuation among specific methods. Good follow-up is essential to ensure users are comfortable with their choice of contraceptive method.
Table 5-1 Contraceptive Failure Rates
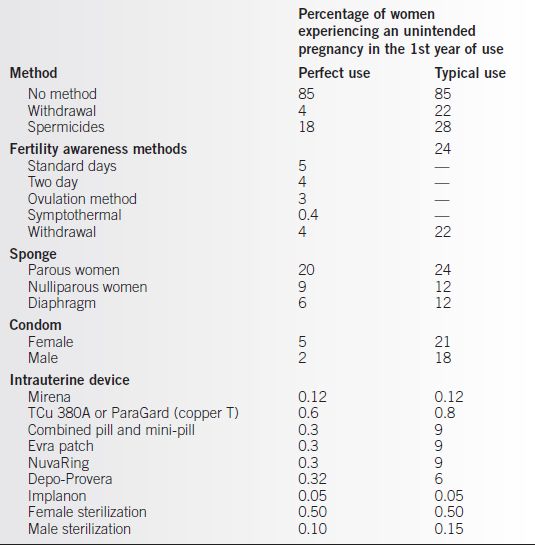
Adapted from Trussell J. Contraceptive efficacy. In: Hatcher RA, Trussell J, Stewart F, et al., eds. Contraceptive technology. 20th ed. New York: Ardent Media, 2011.
Evaluation
• Although no perfect contraceptive exists, a wide range of methods are available, and almost every user and couple should be able to find a method that suits their needs.
• To choose a contraceptive method that will be effective for them, couples and individuals need information and advice from their medical care provider. This should include method-specific information on:
• Efficacy
• Side effects
• Risks
• Potential benefits
• Convenience
• Expense
• Providers need to evaluate prospective users for contraindications (2).
Hormonal Contraception
Combined Oral Contraceptives (COC)
Background
• Oral contraceptives prevent pregnancy primarily by inhibiting ovulation, although thickening of cervical mucus, endometrial changes, and reduction of tubal motility may also contribute.
• Combination hormone preparations:
• With combination therapy, a tablet containing both an estrogen and a progestin is taken for 21 days, beginning between the 1st and 5th days of the menstrual cycle or on the first Sunday after the menses begin.
• The primary antifertility effect is mediated by the progestin, which prevents ovulation and effects changes in the endometrium and the cervical mucus.
• The estrogen is added principally to decrease the number of days of vaginal bleeding experienced by the patient.
• Triphasic pills contain different doses of progestin, and in some cases estrogen, in each 7-day segment of the cycle and allow a reduction in hormone dose from the levels in the monophasic pills.
• A formulation, Seasonale, is approved in the United States to reduce a women’s menstrual cycle to four times a year instead of the usual 13 times. Previously, women were instructed to continue taking the active birth control pills without placebo to delay their periods.
• Combined oral contraceptives (COCs) do not protect against STI/HIV. If there is a risk of STI/HIV, consistent and correct condom use is recommended, either alone or with another contraceptive method.
Effect on Various Organ Systems
• In addition to their effect on fertility, oral contraceptives exert effects on many other organ systems. Some of these effects may be beneficial (11), while others may be unwanted. For example, certain organ function tests are altered substantially by oral contraceptives, thus complicating the diagnosis of disease in these organs during oral contraceptive use.
• Contraindications to the use of contraceptive pills frequently are based on the drug’s effects on a particular organ system. The side effects of the pills may result from an undesirable action of the drug on certain organs.
Effect on Reproductive Organs
• COCs prevent ovulation, thus decreasing the incidence of functional ovarian cysts, although there is less suppression of ovarian cysts with the low-dose monophasic and multiphasic pills. COCs are an effective treatment for patients with polycystic ovarian syndrome (PCOS) (3).
• Breast effects:
• Oral contraceptives decrease the incidence of benign breast disease.
• There is no evidence of an overall increase in the risk of breast cancer with pill use (4). Among users with a family history of breast cancer, there is no increased risk of breast cancer compared with non-COC users with a family history of breast cancer.
• Breast tenderness is a well-recognized side effect of oral contraceptives, caused primarily by the estrogen component.
• If given during the postpartum period, combination (but not progestin-only) pills may decrease milk production, as well as the milk’s protein and fat content.
Effect on Other Endocrine Organs
• Estrogen increases the amount of circulating binding globulins, thus increasing the total amount of bound circulating hydrocortisone and thyroxine. Because these increases are in the bound (inactive) fraction of the hormone, no recognized change occurs in either adrenal or thyroid function.
• Although low-dose oral contraceptives may cause a slight decrease in glucose tolerance, there is no evidence of an increased risk of developing overt diabetes.
• Women with a history of gestational diabetes may safely use oral contraceptives.
• Women under age 35 with overt diabetes of less than 20 years’ duration, without vascular disease, may safely use oral contraceptives with appropriate monitoring (2).
Effect on Other Organ Systems
• Minimal changes in certain blood-clotting factors have been reported with low-dose oral contraceptives. There is a small increase in the risk of venous thromboembolism (VTE), primarily in the 1st year of use.
• The hypertension that occurs in a small number of patients taking oral contraceptives may be mediated through changes in the renin–angiotensin system. The blood pressure usually returns to normal when the pills are discontinued.
• Liver effects:
• Some liver function tests may show elevated levels, but this change is of no known clinical significance.
• COCs are metabolized by the liver, and their use may adversely affect women whose liver is already compromised. COC use has not been shown to inhibit the action of other drugs, but some medications can clinically interfere with the action of COCs, including barbiturates, sulfonamides, cyclophosphamide, and rifampin. While evidence of a clear negative impact on the contraceptive effectiveness of COCs is lacking, it is prudent to suggest use of an alternative or additional contraceptive method when using these other medications.
• Cholestasis may occur with use of oral contraceptives, just as it may occur during pregnancy.
• Hyperpigmentation of the face in a butterfly distribution (melasma) occurs in a few patients taking oral contraceptives. This may not disappear completely after discontinuation of pill use.
Side Effects
• Because oral contraceptives are potent agents capable of exerting effects on virtually all organ systems, it is not surprising that side effects are common. Most are merely annoying, but a few are life threatening.
• Counseling patients who choose oral contraception must include a description of the possible serious complications and an estimation of the risk for the patient.
Major Considerations
Vascular Complications
• Studies have shown a two- to sevenfold increased risk of VTE in users of combined hormonal contraceptives (CHCs) compared with women who do not use CHCs (5).
• COCs containing third-generation progestogens (desogestrel, norgestimate, and gestodene) or the progestin drospirenone have a greater risk of VTE (1.5- to 3-fold increase over levonorgestrel).
• The most important risk factor for thromboembolism appears to be inherited thrombophilia. Routine screening is NOT appropriate because of the rarity of the conditions and the high cost of screening (6).
• Recent meta-analysis studies have shown an increased risk of stroke and myocardial infarction (MI) even with current low-dose OC formulations containing second- and third-generation progestogens. Age, apart from cardiovascular risk factors, appears to be the predominant factor in this increased risk, so use of low-dose OCs in healthy women should not increase the incidence of these adverse outcomes (7).
• Women with underlying vascular disease have an increased risk of arterial thrombosis with COC use and should not use COCs. Because of the association of vascular-type headaches and stroke, women who develop migraine headaches associated with aura also should not use COCs (2).
• The risk of developing a blood clot from a CHC is higher than when not using CHC but still remains lower than the risk of developing blood clots in pregnancy and in the postpartum period (8).
Liver Tumors
• Oral contraceptive use has been associated with the development of a rare liver tumor, benign hepatocellular adenoma. These tumors appeared to be related to the estrogenic component and are related to both historically higher-dose formulations and extended duration of use of oral contraceptives. More recent data show an emerging role of obesity as a contributing factor rather than current COC formulations (9).
• Although some of the tumors have been resected successfully, they will usually regress spontaneously if oral contraceptives are discontinued.
Breast Cancer
• Although the majority of studies to date, including the large Cancer and Steroid Hormone study (10), show no overall increase in breast cancer risk, several studies have suggested a small increased risk of premenopausal breast cancer among current oral contraceptive users.
• A study that analyzed various formulations of oral contraceptives found no evidence that breast cancer risk varies significantly by OC formulation, and no specific OC formulation was associated with a significantly increased risk of breast cancer (4).
Endometrial and Ovarian Cancer
• Oral contraceptive use has been shown to decrease the incidence of both endometrial and ovarian cancers (8). Almost all of the studies on both these cancers and oral contraceptive use have shown a protective effect.
• For women who have used oral contraceptives for at least 1 year, the relative risk of endometrial cancer is 0.5.
• The relative risk of ovarian cancer is 0.6, and a protective effect is seen with as little as 3 to 6 months of use.
• For both cancers, the protective effect continues for at least 15 to 20 years after the last use of oral contraceptives.
Cervical Cancer
• The association of oral contraceptive use and cervical cancer remains controversial. A large systematic review of the literature showed a significantly increased risk with more than 5 years of use by women who are positive for human papillomavirus (12).
• Most studies of oral contraceptive use and the risk of cervical dysplasia, carcinoma in situ, and invasive cancer have found a small increase in risk. Unfortunately, many of the studies failed to control for at least a few of the known risk factors related to the risk of cervical cancer, such as number of sexual partners, age at first intercourse, and smoking history.
Hypertension
• Oral contraceptives appear to be associated with a small increase in the incidence of hypertension.
• In most women who experience hypertension with oral contraceptives, the rise in blood pressure is small.
• A few women may experience a more severe elevation in blood pressure, presumably as a result of a derangement in the renin–angiotensin system.
• Among women with preexisting hypertension, smoking, along with oral contraceptive use, is associated with an increased risk of cardiovascular disease, particularly in women over the age of 35. Women over the age of 35 who smoke should not use COCs. Nonsmoking women with well-controlled hypertension can usually use oral contraceptives safely (2).
• A history of pregnancy-induced hypertension is not a contraindication to oral contraceptive use.
• Blood pressure monitoring is important for all oral contraceptive users, particularly in the first few months of use.
Minor Side Effects
• Certain minor but troublesome side effects are associated with oral contraceptive use. These symptoms include breakthrough bleeding, nausea, vomiting, and weight gain (13).
Intermenstrual Bleeding
• Intermenstrual bleeding is common during the first 3 months of oral contraceptive use and will usually abate by the 4th month of use.
• After the first 3 months of use, bleeding that occurs early in the cycle may be related to the low estrogen content of the pill, and bleeding that occurs late in the cycle may be the result of a deficient progestin content.
• Although the bleeding may disappear spontaneously, switching to a different pill with an appropriate balance of hormones will usually solve the problem.
Nausea and Vomiting
• Nausea and vomiting are related to the estrogen content of the pill. They occur most commonly during the first few months of pill use.
• The symptoms may be eliminated by switching to a pill with a lower estrogen content or by simply having the patient take the pill consistently with the evening meal or at bedtime.
Weight Gain
• Weight gain is reported frequently with oral contraceptives, although scientific evidence does not support a causal association between COCs or a combination skin patch and weight change (13).
• Most comparisons of different combination contraceptives show no substantial difference in weight changes.
Continuous Use and Postpill Amenorrhea
• Use of OCPs in a continuous manner, where patients remain on active hormonal contraceptive pills without a week of placebo pills, is a treatment for primary dysmenorrhea or endometriosis and may induce amenorrhea throughout the treatment period (14).
• The occurrence of post-OC amenorrhea has been estimated to range from 0.2% to 3.1% depending on the definition of the duration of amenorrhea; however, there does not seem to be any significant difference between the occurrence of spontaneous and post-OC amenorrhea.
• A diagnostic investigation is indicated if amenorrhea continues for 6 months or more or is associated with galactorrhea.
Contraindications
Absolute Contraindications
• Use of the combination oral contraceptives is not recommended if any of the following is present (2):
• Known thrombophilia, history of or current thrombophlebitis, pulmonary embolus, cerebral hemorrhage, coronary artery disease, or valvular heart disease with complications
• Current markedly impaired liver function
• History of or current hepatic adenoma or carcinoma
• Known or suspected carcinoma of the breast or other estrogen-dependent tumor
• Pregnancy
• Diabetes with vascular disease or diabetes of greater than 20 years’ duration
• Lactation less than 6 weeks postpartum
• Migraine headaches with aura
• Age over 35 and smoking greater than 15 cigarettes per day
• Uncontrolled hypertension
• Surgery or orthopedic injury requiring prolonged immobilization
Caution
• Caution should be exercised in prescribing oral contraceptives for patients who have the following conditions:
• Hypertension
• Gallbladder disease
• Diabetes
• Migraine without aura
• Cholestasis during pregnancy
• Cardiac or renal disease
• Planned elective major surgery
• Family history of hyperlipidemia or MI in a parent or sibling younger than 50 years
• Undiagnosed genital bleeding
• Sickle cell disease or sickle C disease
• Active gallbladder disease
Choice of Medication
Initial Prescription
• When a patient has decided to take oral contraceptives, a pill with as low a dose of estrogen as the patient will tolerate should be prescribed because most of the serious complications are related to the estrogen component.
• When prescribing the pill, be certain that the patient does not have an absolute contraindication. If a relative contraindication exists, inform the patient and encourage her to consider other contraceptive methods (2).
• The patient who is just beginning to use oral contraception should be given sufficient medication for a 3-month period, and she should be encouraged to read the package insert. Any symptom of thrombophlebitis, pulmonary embolism, coronary occlusion, cerebrovascular accident, or an eye problem should be reported immediately.
• The patient may start using the pill the day she receives the medication (Quick Start) or wait until the start of her next period. Evidence suggests that neither the risk of inadvertently starting COCs in a woman who is pregnant nor the risk of pregnancy after COC initiation are affected by the cycle day on which COCs are started. While starting CHCs via Quick Start may initially increase continuation compared with more conventional starting strategies, evidence suggests that this difference disappears over time (15).
• If the patient reports no problems, she should be seen at 3 months, at which time
• A history should be obtained regarding headaches, blurred vision, and leg, chest, or abdominal pain.
• Blood pressure should be checked.
• The patient should be encouraged to raise any concerns that may affect her willingness to continue with her current choice.
• The patient should then be seen at 12-month intervals, at which time a history of possible complications and blood pressure are taken, and, if indicated, a brief physical examination, including breast, abdominal, and pelvic examination as well as health screening tests as indicated.
Nonoral Combined Hormonal Contraceptives
Transdermal Patch
Background
• The Ortho Evra patch contains ethinyl estradiol and the progestin norelgestromin. The patch is used weekly for 3 weeks on with 1 week off.
• The patch has the potential for improved compliance as a woman does not have to remember to take a pill daily. Studies have demonstrated an improvement of 5% to 15% perfect compliance rate with the patch over oral contraceptives (16).
Effects
• Efficacy, contraindications, side effects, and complications are similar to those with COCs. Skin irritation from the patch also occurs in up to 20% of users.
Complications
• Concern has arisen about the patch’s potential for a higher risk of cardiovascular complications because of a higher steady state concentration and area under the curve (AUC), and a lower peak concentration of ethinyl estradiol in comparison with 35 μg oral contraceptives.
• There appears to be a slightly higher increased risk of thrombotic events from use of the patch than with COCs, but, as with COCs, this risk is the risk attributed to age and other cardiovascular risk factors (7).
Vaginal Ring
Background
• The NuvaRing is a 54-mm flexible vaginal ring that contains ethinyl estradiol and etonogestrel. The ring is placed in the vagina for 3 weeks and then removed for 1 week.
• Progering is a progesterone vaginal ring currently available in Latin America for use by breast-feeding women. One ring may be used for up to 3 months.
• Although not recommended, the ring may be removed during sexual intercourse for a period of up to 2 hours.
Effects
• The efficacy, contraindications, side effects, and complications of the vaginal ring are similar to those with COCs.
• Cycle control, compliance, and continuation rates are equal or higher than with COCs (17).
Complications
• Expulsion of the ring is reported by 4% to 20% of women.
• Approximately 6% of women using the ring complain of vaginal discharge.
Progestin-Only Hormonal Contraception
Progestin-Only Pill
Background
• The progestin-only pill (or mini-pill) may be appropriate for patients who have a contraindication to an estrogen-containing pill. In the United States, norethindrone (Camila) is the only currently available progestin-only pill, while in Europe, both norethindrone (Micronor) and desogestrel (Cerazette) are available as progestinonly pills.
• The progestin-only pill is recommended over regular birth control pills for women who are breast-feeding because the min-pill does not affect milk production.
Effects
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


