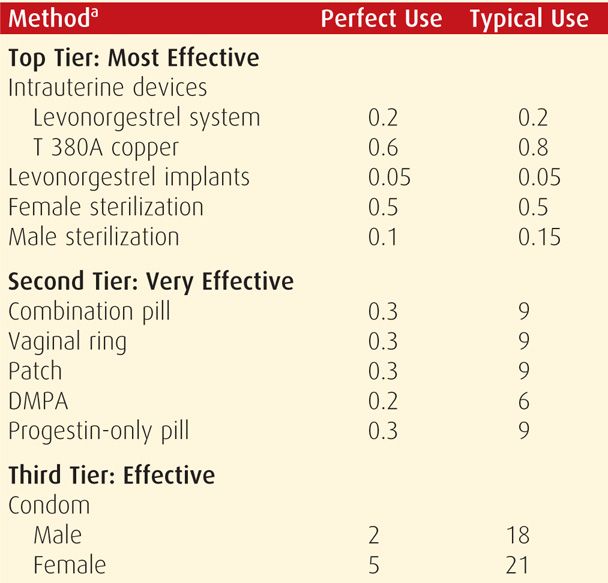
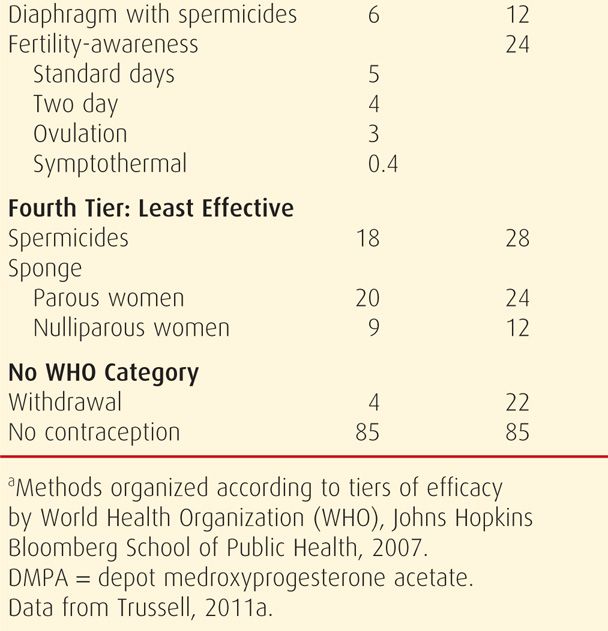
TABLE 38-2. Contraceptive Failure Rates During the First Year of Method Use in Women in the United States
Unfortunately, no contraceptive method is completely without side effects. That said, an important tenet is that contraception usually poses less risk than pregnancy. During appropriate method selection, underlying patient health conditions should be known. Some disorders or the medications used for their treatment can increase the risk of some contraceptives. The WHO (2010) has provided evidence-based guidelines, termed Medical Eligibility Criteria, for the use of all highly effective reversible contraceptive methods by women with various health conditions. Individual countries have subsequently modified these. The United States Medical Eligibility Criteria (US MEC) was published in the United States by the Centers for Disease Control and Prevention (CDC) (2010b), and its use is encouraged by the American College of Obstetricians and Gynecologists (2011b). Shown on page 698, the CDC (2011, 2012) has since added updates to reflect changes for women at high risk for human immunodeficiency virus (HIV) infection and for women in the puerperium. The US MEC guidelines and these updates, as well as a free smart-phone application of the guidelines, are available at the CDC website: http://www.cdc.gov/reproductivehealth/UnintendedPregnancy/USMEC.htm.
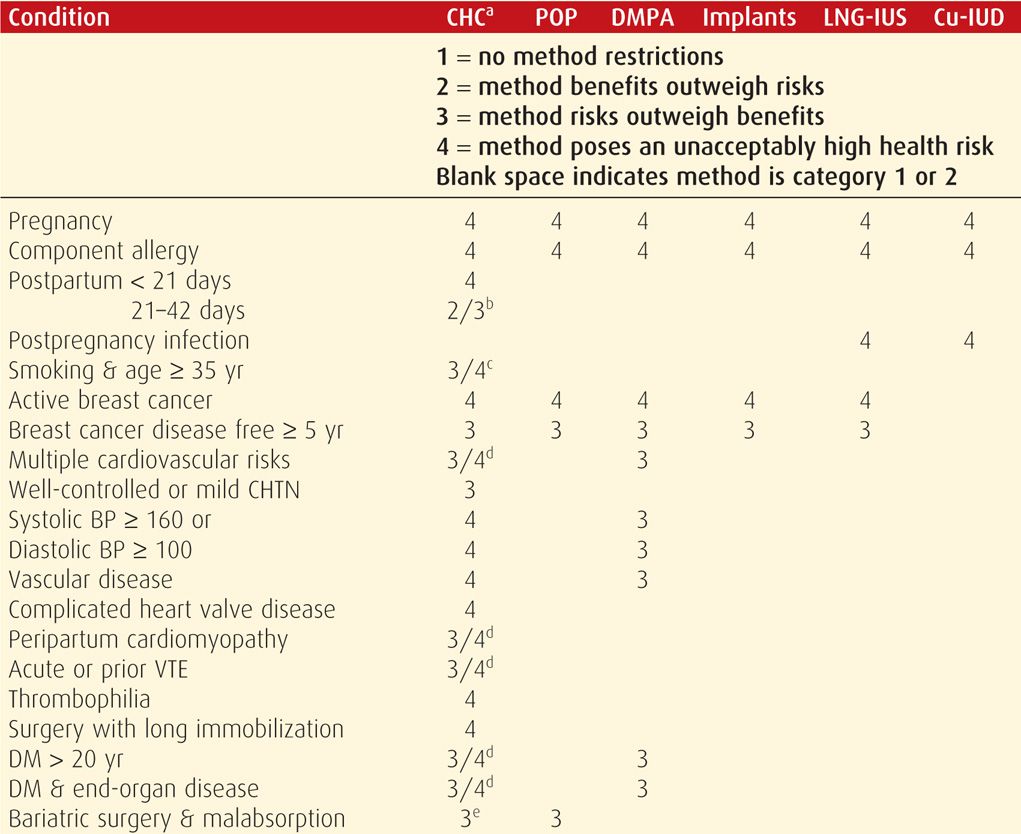
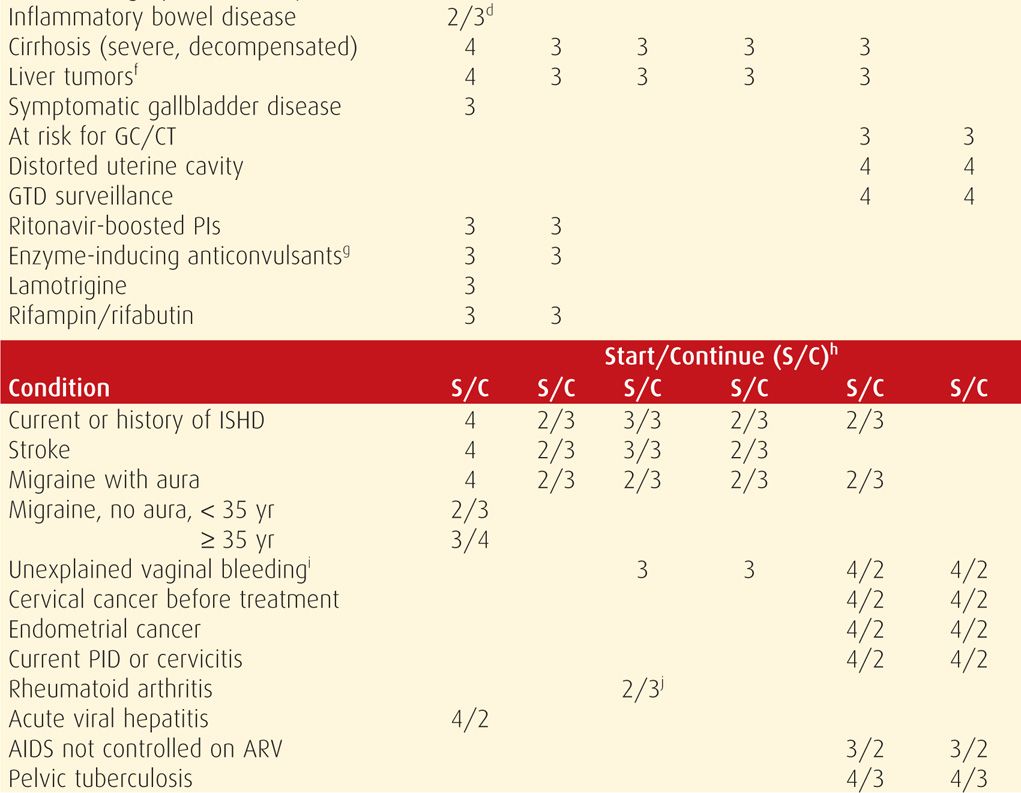
In the US MEC, reversible contraceptive methods are organized into six groups by their similarity: combination hormonal contraceptives (CHCs), progestin-only pills (POPs), depot medroxyprogesterone acetate (DMPA), implants, levonorgestrel-releasing intrauterine system (LNG-IUS), and copper intrauterine devices (Cu-IUDs). For a given health condition, each method is categorized 1 through 4. The score describes a method’s safety profile for a typical woman with that condition: (1) no restriction of method use, (2) method advantages outweigh risks, (3) method risks outweigh advantages, and (4) method poses an unacceptably high health risk.
Alternatively, depending on the underlying disorder or patient desire, male or female sterilization may be a preferred or recommended permanent contraceptive method. These options are fully discussed in Chapter 39 (p. 720).
LONG-ACTING REVERSIBLE CONTRACEPTION: INTRAUTERINE DEVICES
These are the most commonly used method of reversible contraception worldwide, and in the United States, nearly 4 percent of women select this method (d’Arcanques, 2007; Jones, 2012). Fortunately, contraindications to IUD use are few.
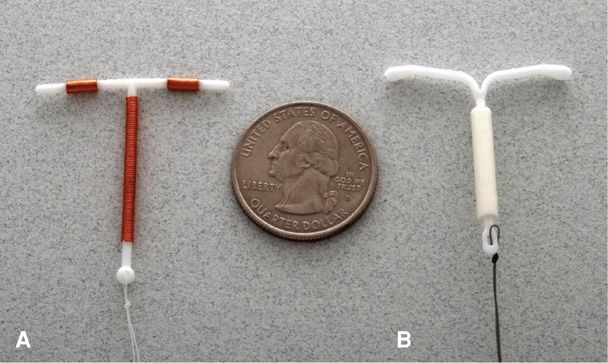
Intrauterine devices (IUDs) that are chemically inert are composed of nonabsorbable materials. The three IUDs currently approved for use in the United States are chemically active and have continuous elution of copper or a progestin. Of these, there are two different levonorgestrel-releasing intrauterine systems—Mirena and Skyla (Fig. 38-1). Each releases the progestin into the uterus at a relatively constant rate, which reduces systemic effects. Their T-shaped radiopaque frames have a stem wrapped with a cylinder reservoir that contains the levonorgestrel. There are two trailing brown strings attached to the stem (Bayer Healthcare Pharmaceuticals, 2013a,b). Mirena is currently approved for 5 years of use following insertion, however, some evidence supports its efficacy for 7 years (Thonneau, 2008). Skyla is approved for 3 years of use. It has slightly smaller overall dimensions than its counterpart and was sized to more appropriately fit a nulliparous uterus (Gemzell-Danielsson, 2012). It can also be differentiated from Mirena visually and sonographically by a silver ring near the junction of the device’s stem and arms.
FIGURE 38-1 Intrauterine devices (IUDs). A. ParaGard T 380A copper IUD. B. Mirena levonorgestrel-releasing intrauterine system.
The third device is the T 380A IUD, named ParaGard. It has a polyethylene and barium sulfate T-shaped frame wound with copper, and two strings extend from the stem base. Originally blue, the strings are now white. It is currently approved for 10 years of use following insertion (Teva Women’s Health, 2011).
In addition to these three currently marketed, women may retain discontinued brands of IUD. A Lippes Loop has two “S” shapes stacked one on the other. The Dalkon Shield has a crab form, whereas a Copper 7 mirrors that number. Progestasert is an early T-shaped progestin-releasing IUD. Last, various metal-eluting ring-shaped devices are common in Asia.
 Contraceptive Action
Contraceptive Action
All these IUDs are effective. Failure rates are well below 1 percent and similar overall to those of tubal sterilization (American College of Obstetricians and Gynecologists, 2013a; Thonneau, 2008; Trussell, 2011b). Their mechanisms have not been precisely defined, but prevention of fertilization is now favored. Within the uterus, an intense local endometrial inflammatory response is induced, especially by copper-containing devices. Cellular and humoral components of this inflammation are expressed in endometrial tissue and in fluid filling the uterine cavity and fallopian tubes. These lead to decreased sperm and egg viability (Ortiz, 2007). Also, in the unlikely event that fertilization does occur, the same inflammatory actions are directed against the blastocyst. The endometrium is transformed into a hostile site for implantation. With the copper IUD specifically, copper levels increase in the cervical mucus of users and decrease sperm motility and viability (Jecht, 1973). With the LNG-IUS, in addition to an inflammatory reaction, long-term progestin release leads to endometrial atrophy, which hinders normal implantation. Moreover, progestins create scant viscous cervical mucus that obstructs sperm motility. The LNG-IUS may also inconsistently release sufficient progestin to inhibit ovulation, although this is a lesser effect than its local actions.
 Method-Specific Adverse Effects
Method-Specific Adverse Effects
Several method-specific complications may follow IUD insertion and include uterine perforation, device expulsion, menstrual changes, infection, and miscarriage if pregnancy occurs. Also, in the past, IUDs were perceived to increase the risk of ectopic pregnancy. However, this has been clarified. Specifically, IUDs are so effective as contraceptives that they lower the absolute number of ectopic pregnancies by half compared with the rate in noncontracepting women (World Health Organization, 1985, 1987). But, the IUD mechanisms of action are more effective in preventing intrauterine implantation. Thus, if an IUD fails, a higher proportion of pregnancies are likely to be ectopic (Backman, 2004; Furlong, 2002).
Perforation
During uterine sounding or IUD insertion, the uterus may be perforated, which is identified by the tool traveling further than the expected uterine length based on initial bimanual examination. Rates approximate 1 per 1000 insertions, and risks include puerperal insertion, lactation, provider inexperience, and extremes of uterine flexion (Harrison-Woolrych, 2003). Although devices may migrate spontaneously into and through the uterine wall, most perforations occur, or at least begin, at the time of insertion.
With the more common fundal perforation, bleeding typically is minimal due to myometrial contraction around the site. If no brisk or persistent bleeding is noted from the os following instrument removal, then patient observation alone is reasonable. Rarely, lateral perforations may lead to uterine artery laceration and brisk bleeding, which may prompt laparoscopy or laparotomy for control. Following any perforation, although this is not firmly evidence-based, a single dose of broad-spectrum antibiotic may mitigate infection.
Lost Device
Expulsion of an IUD from the uterus is most common during the first month. Thus, women should be examined approximately 1 month following IUD insertion, usually after menses, to identify the tails trailing from the cervix. Following this, a woman should be instructed to palpate the strings each month after menses.
If the tail of an IUD cannot be visualized, the device may have been expelled, may have perforated the uterus, or may be malpositioned. Some have found that IUD perforation, rotation, or embedding may cause pain or bleeding (Benacerraf, 2009; Moschos, 2011). Alternatively, the device may be normally positioned with its tail folded within the endocervical canal or uterine cavity. To investigate, after excluding pregnancy, a cytological brush can be twirled within the endocervical canal to entangle the strings and bring them gently into the vagina. If unsuccessful, the uterine cavity is probed gently with a Randall stone clamp or with a specialized rod with a terminal hook to retrieve the strings.
It should never be assumed that the device has been expelled unless it was seen. Thus, if tails are not visible and the device is not felt by gentle probing of the uterine cavity, transvaginal sonography can be used to ascertain if the device is within the uterus. Although traditional sonography will document IUD position adequately in most cases, three-dimensional sonography offers improved visualization, especially with the levonorgestrel-releasing IUD (Moschos, 2011). If sonography is inconclusive or if no device is seen, then a plain radiograph of the abdominopelvis is taken. Computed tomography (CT) scanning or less commonly, magnetic resonance (MR) imaging is an alternative (Boortz, 2012). It is safe to perform MR imaging at 1.5 and 3 Tesla (T) with an IUD in place (Pasquale, 1997; Zieman, 2007).
A device may penetrate the muscular uterine wall to varying degrees. Those with a predominantly intrauterine location are typically managed by hysteroscopic IUD removal. In contrast, devices that have nearly or completely perforated through the uterine wall are more easily removed laparoscopically. Chemically inert devices usually are removed easily from the peritoneal cavity. An extrauterine copper-bearing device, however, frequently induces an intense local inflammatory reaction and adhesions. Thus, copper IUDs may be more firmly adhered. Laparotomy may be necessary, and bowel preparation is considered. Perforations of large and small bowel and bladder and bowel fistulas have been reported remote from insertion (Lyon, 2012; Mascarenhas, 2012; Zeino, 2011).
Menstrual Changes
Women who choose the copper IUD should be informed that dysmenorrhea and menorrhagia may develop. These are often treated with nonsteroidal antiinflammatory drugs (NSAIDs) (Grimes, 2006). Heavy bleeding may cause iron deficiency anemia, for which oral iron salts are given (Hassan, 1999).
With the LNG-IUS, women are counseled to expect irregular spotting for up to 6 months after placement, and thereafter to expect monthly menses to be lighter or even absent (Bayer HealthCare Pharmaceuticals, 2013a). Specifically, the Mirena device is associated with progressive amenorrhea, which is reported by 30 percent of women after 2 years and by 60 percent after 12 years (Ronnerdag, 1999). This is often associated with improved dysmenorrhea.
Infection
The device-related infection risk is increased only during the first 20 days following insertion (Farley, 1992). Of those who develop an infection during this time, most usually have an unrecognized coexistent cervical infection. Accordingly, women at risk for sexually transmitted diseases (STDs) should be screened either before or at the time of IUD insertion (Centers for Disease Control and Prevention, 2010a; Sufrin, 2012). That said, device insertion need not be delayed while awaiting Neisseria gonorrhoeae, Chlamydia trachomatis, or PAP screening results in asymptomatic women. If these bacteria are found and the patient is without symptoms, then the IUD may remain and treatment prescribed as detailed in Chapter 65 (p. 1269). Importantly, routine antimicrobial prophylaxis before insertion is not recommended (Grimes, 2001; Walsh, 1998). Moreover, the American Heart Association does not recommend infective endocarditis prophylaxis with insertion (Wilson, 2007).
After these first 3 weeks, infection risk is not increased in IUD users who would otherwise be at low risk of STDs. Correspondingly, IUDs appear to cause little, if any, increase in the risk of infertility in these low-risk patients (Hubacher, 2001). For these reasons, the American College of Obstetricians and Gynecologists (2011a, 2012a) recommends that women at low risk for STDs, including adolescents, are good candidates for IUDs (Table 38-3). The IUD is also safe and effective in HIV-infected women and may be used in others who are immunosuppressed (Centers for Disease Control and Prevention, 2012).
If infection does develop, it may take several forms and typically requires broad-spectrum antimicrobials. Septic abortion mandates immediate uterine evacuation in addition to antimicrobials. Pelvic inflammatory disease (PID) without abscess is treated with systemic antibiotics. There are theoretical concerns that a coexistent IUD might worsen the infection or delay resolution. Although a provider may choose to remove an IUD in this setting, some evidence also supports allowing a device to remain during treatment in those hospitalized with mild or moderate PID (Centers for Disease Control and Prevention, 2010b; Tepper, 2013). Last, tuboovarian abscess may develop with PID and is treated aggressively with intravenous broad-spectrum antibiotics and IUD removal.
In addition to these infections, Actinomyces israelii is a gram-positive, slow-growing, anaerobic indigenous vaginal bacterium that rarely causes infection with abscess formation (Persson, 1984). Some have found it more frequently in the vaginal flora or on the Pap smears of IUD users (Curtis, 1981; Fiorino, 1996). Current recommendations advise that an asymptomatic woman may retain her IUD and does not require antibiotic treatment (American College of Obstetricians and Gynecologists, 2013c; Lippes, 1999; Westhoff, 2007a). However, if signs or symptoms of infection develop in women who harbor Actinomyces species, then the device should be removed and antimicrobial therapy instituted. Early findings with infection include fever, weight loss, abdominal pain, and abnormal vaginal bleeding or discharge. Actinomyces species are sensitive to antimicrobials with gram-positive coverage, notably the penicillins.
Pregnancy with an IUD
For women who become pregnant while using an IUD, ectopic pregnancy should be excluded (Chap. 19, p. 379). With intrauterine pregnancy, until approximately 14 weeks’ gestation, the tail may be visible through the cervix, and if seen, it should be grasped and the IUD removed by gentle outward traction. This action reduces complications such as subsequent abortion, chorioamnionitis, and preterm birth (Brahmi, 2012; Kim, 2010). Tatum and coworkers (1976) reported an abortion rate of 54 percent with the device left in place compared with a rate of 25 percent if it was promptly removed.
If the tail is not visible, attempts to locate and remove the device may result in abortion. However, some practitioners have successfully used sonography to assist in device removal in cases without visible strings (Schiesser, 2004). After fetal viability is reached, it is unclear whether it is better to remove an IUD whose string is visible and accessible or to leave it in place. There is no evidence that fetal malformations are increased with a device in place (Tatum, 1976).
Second-trimester miscarriage with an IUD in place is more likely to be infected (Vessey, 1974). Sepsis may be fulminant and fatal. Pregnant women with a device in utero who demonstrate any evidence of pelvic infection are treated with intensive antimicrobial therapy and prompt uterine evacuation. Because of these risks, a woman should be given the option of early pregnancy termination if the device cannot be removed early in pregnancy. Last, in women who give birth with a device in place, appropriate steps should be taken at delivery to identify and remove the IUD.
 Insertion
Insertion
Timing
Immediately following miscarriage, surgical abortion, or delivery, an IUD may be inserted in the absence of infection. Also, Shimoni and colleagues (2011) describe “immediate” insertion 1 week after medical abortion. The risk of IUD expulsion is slightly higher if it is placed immediately following any of these recent pregnancies. That said, the advantages of preventing future unplanned pregnancies appear to outweigh this (Bednarek, 2011; Chen, 2010; Grimes, 2010a; Steenland, 2011).
Insertion techniques depend on uterine size. After first-trimester evacuation, the IUD can be placed using the manufacturer’s standard instructions. If the uterine cavity is larger, the IUD can be placed using ring forceps with sonographic guidance (Stuart, 2012). Immediately following vaginal or cesarean delivery, an IUD can be placed by hand or with an instrument (Grimes, 2010b). To reduce expulsion rates and to minimize the perforation risk, some may choose to wait for complete involution—at least 6 weeks after delivery. Women delivered at Parkland Hospital are seen 3 weeks postpartum, and IUDs are inserted 6 weeks postpartum or sooner if involution is complete.
For placement not related to pregnancy, insertion near the end of normal menstruation, when the cervix is usually softer and somewhat more dilated, may be easier and at the same time may exclude early pregnancy. But, insertion is not limited to this time. For the woman who is sure she is not pregnant and does not want to be pregnant, insertion is done at any time.
Technique
Before insertion, several procedural steps are carried out. Any contraindications are identified. If there are none, the woman is counseled and written consent obtained. An oral NSAID, with or without codeine, can be used to allay cramps (Karabayirli, 2012). Strong evidence does not support an advantage to supplemental misoprostol, intrauterine or cervical canal instillation of lidocaine, or paracervical blockade to lessen insertional pain (McNicholas, 2012; Nelson, 2013; Swenson, 2012). Bimanual pelvic examination is performed to identify uterine position and size. Abnormalities are evaluated, as they may contraindicate insertion. Mucopurulent cervicitis or significant vaginitis should be appropriately treated and resolved before IUD insertion.
The cervical surface is cleansed with an antiseptic solution, and sterile instruments and a sterile IUD should be used. A tenaculum is placed on the cervical lip, and the canal and uterine cavity are straightened by applying gentle traction. The uterus is then sounded to identify the direction and depth of the uterine cavity. Specific steps of ParaGard and Mirena insertion are shown in Figures 38-2 and 38-3 and outlined in their respective package inserts.
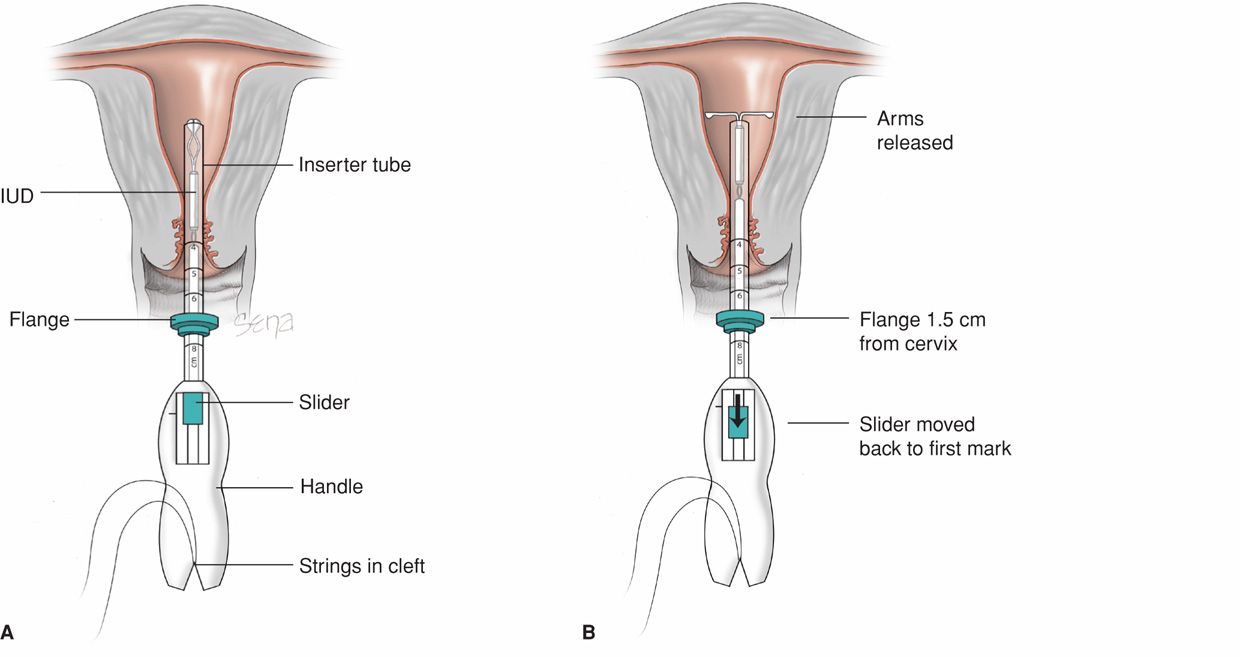
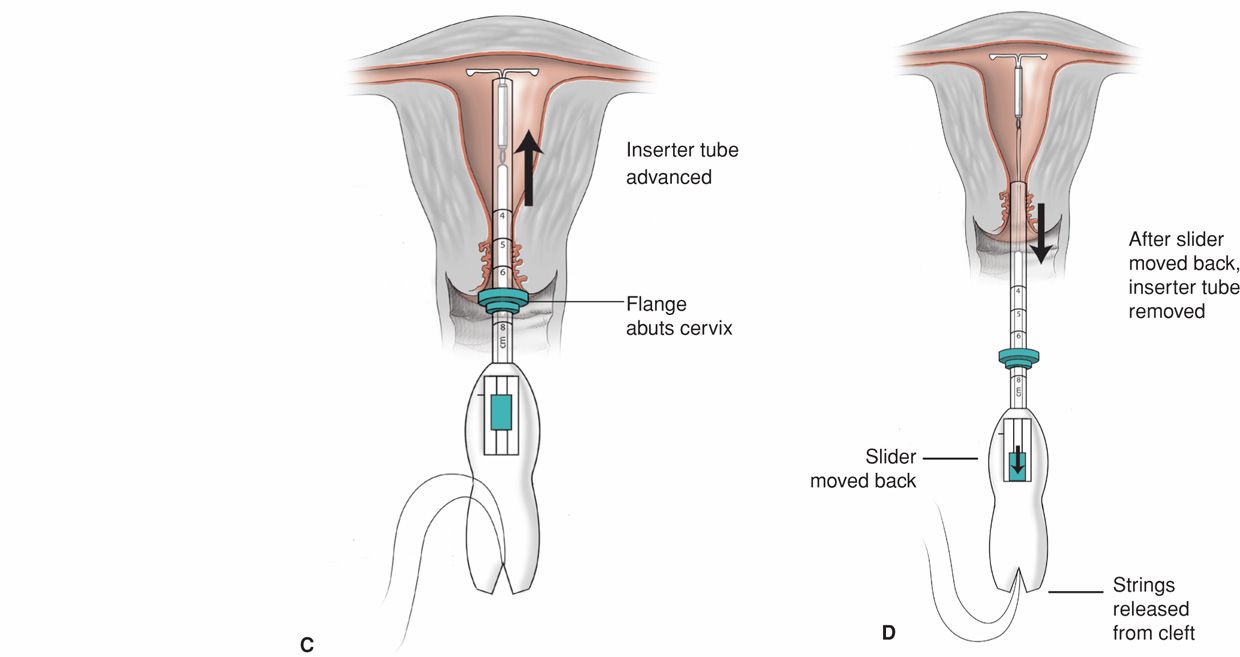
FIGURE 38-2 Insertion of the Mirena intrauterine system. Initially, threads from behind the slider are first released to hang freely. The slider found on the handle should be positioned at the top of the handle nearest the device. The IUD arms are oriented horizontally. A flange on the outside of the inserter tube is positioned from the IUD tip to reflect the depth found with uterine sounding. A. As both free threads are pulled, the Mirena IUD is drawn into the inserter tube. The threads are then tightly fixed from below into the handle’s cleft. In these depictions, the inserter tube has been foreshortened. The inserter tube is gently inserted into the uterus until the flange lies 1.5 to 2 cm from the external cervical os to allow the arms to open. B. While holding the inserter steady, the IUD arms are released by pulling the slider back to reach the raised horizontal mark on the handle, but no further. C. The inserter is then gently guided into the uterine cavity until its flange touches the cervix. D. The device is released by holding the inserter firmly in position and pulling the slider down all the way. The threads will be released automatically from the cleft. The inserter may then be removed, and IUD strings trimmed.
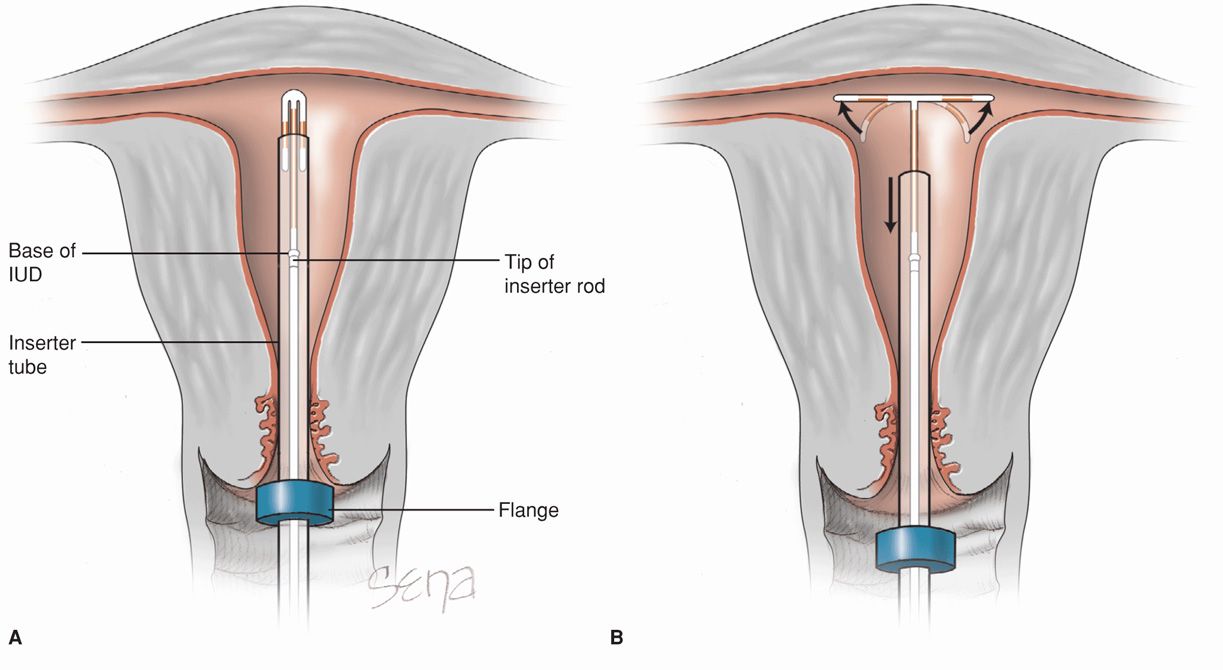
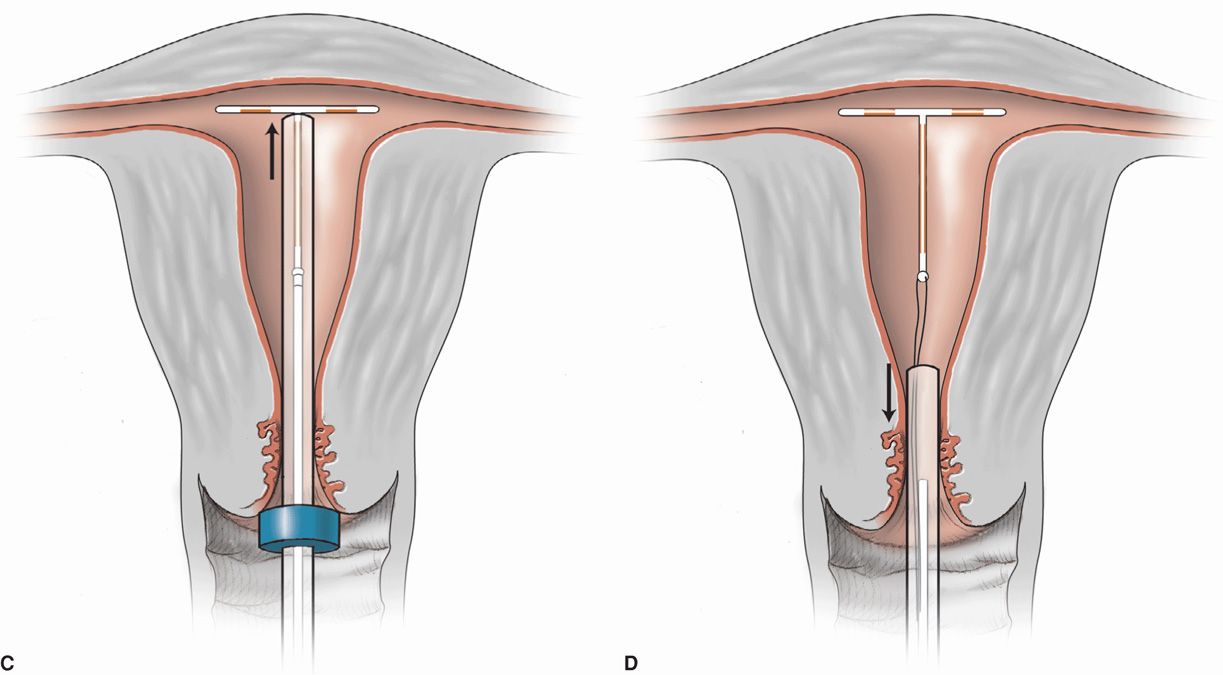
FIGURE 38-3 Insertion of ParaGard T 380A. The uterus is sounded, and the IUD is loaded into its inserter tube not more than 5 minutes before insertion. A blue plastic flange on the outside of the inserter tube is positioned from the IUD tip to reflect uterine depth. The IUD arms should lie in the same plane as the flat portion of the oblong blue flange. A. The inserter tube, with the IUD loaded, is passed into the endometrial cavity. A long, solid, white inserter rod abuts the base of the IUD. When the blue flange contacts the cervix, insertion stops. B. To release the IUD arms, the solid white rod within the inserter tube is held steady, while the inserter tube is withdrawn no more than 1 cm. C. The inserter tube, not the inserter rod, is then carefully moved upward toward the top of the uterus until slight resistance is felt. At no time during insertion is the inserter rod advanced forward. D. First, the solid white rod and then the inserter tube are withdrawn individually. At completion, only the threads should be visible protruding from the cervix. These are trimmed to allow 3 to 4 cm to extend into the vagina.
Following insertion, only the threads should be visible trailing from the cervix. These are trimmed to allow 3 to 4 cm to protrude into the vagina, and their length is recorded. If there is suspicion that the device is not positioned correctly, then placement should be confirmed, using sonography if necessary. If the IUD is not positioned completely within the uterus, it is removed and replaced with a new device. An expelled or partially expelled device should not be reinserted.
LONG-ACTING REVERSIBLE CONTRACEPTION: PROGESTIN IMPLANTS
 Etonogestrel Implant
Etonogestrel Implant
Contraception can be provided by thin, pliable progestin-containing cylinders that are implanted subdermally and release hormone over many years. One of these, Implanon is a single-rod implant with 68 mg of etonogestrel covered by an ethylene vinyl acetate copolymer cover. The implant is placed subdermally on the medial surface of the upper arm 8 to 10 cm from the elbow in the biceps groove and is aligned with the long axis of the arm. It may be used as contraception for 3 years and then replaced at the same site or in the opposite arm (Merck, 2012a).
Implanon is not radiopaque, and a misplaced implant may be identified with sonography using a 10- to 15-MHz linear array transducer (Shulman, 2006). In some cases, MR imaging may be required if supplemental information is needed despite sonography (Correia, 2012). To improve radiologic detection, the manufacturer developed Nexplanon, which is similarly shaped and pharmacologically identical to Implanon but is radiopaque. Additionally, the Nexplanon inserter device is designed to assist with subdermal placement and avert deeper insertions. Both implants are highly effective, and the mechanism of action for progestin-only products is described on page 704 (Croxatto, 1998; Mommers, 2012). Although still approved by the Food and Drug Administration (FDA), Implanon is no longer distributed by the manufacturer.
 Levonorgestrel Implants
Levonorgestrel Implants
The first progestin implants contained levonorgestrel, and systems are still available outside the United States. Jadelle, originally named Norplant-2, provides levonorgestrel and contraception for 5 years through two subdermally implanted Silastic rods. After this time, rods may be removed and if desired, new rods inserted at the same site (Bayer Schering Pharma Oy, 2010). Jadelle is approved by the FDA, however, it is not marketed or distributed in the United States (Population Council, 2013). Sino-implant II is a two-rod implant with the same amount (150 mg) of levonorgestrel and same mechanism of action as Jadelle but provides 4 years of contraception (Shanghai Dahua Pharmaceutical, 2012). Sino-implant II is manufactured in China and approved for use by several countries in Asia and Africa.
Both implant systems are highly effective, and the mechanism of action for progestin-only products is described on page 704. Like the etonogestrel implant, these systems are placed subdermally on the inner arm approximately 8 cm from the elbow and have similar removal steps. Implants vary regarding their insertion technique, and manufacturer instructions should be consulted.
The forerunner of these implants was the Norplant System, which provided levonorgestrel in six Silastic rods implanted subdermally. The manufacturer stopped distributing the system in 2002.
 Method-Specific Adverse Effects
Method-Specific Adverse Effects
Risks that are specific to implants stem mainly from malpositioning. First, branches of the medial antebrachial cutaneous nerve can be injured if the implant or insertion needle is placed too deeply or if exploration for a lost implant is aggressive. Clinically, numbness and paresthesia over the anteromedial aspect of the forearm are noted (Brown, 2012; Wechselberger, 2006). Second, nonpalpable devices are not uncommon and may require radiological imaging for localization as described earlier. If imaging fails to locate an Implanon or Nexplanon implant, etonogestrel blood level determination can be used to verify that the implant is in situ.
 Insertion
Insertion
Timing
For those not currently using hormonal contraception, the etonogestrel implant is ideally inserted within 5 days of menses onset. If inserted later in the cycle, then alternative contraception is recommended for 7 days following placement. With levonorgestrel-releasing implants, contraception is established within 24 hours if inserted within the first 7 days of the menstrual cycle (Sivin, 1997; Steiner, 2010). For transitioning methods, an implant is placed on the day of the first placebo COC pill; on the day that the next DMPA injection would be due; or within 24 hours of taking the last POP (Merck, 2012a). Related to pregnancy, an implant may be inserted before discharge following delivery, miscarriage, or abortion.
Nexplanon Insertion Technique
With the patient lying down, her nondominant arm, forearm, and hand are outstretched on the bed with the inner aspects of each exposed upward, and the elbow is flexed. The insertion site is marked with a sterile pen 8 to 10 cm proximal to the medial condyle of the humerus. A second mark is placed 4 centimeters proximally and delineates the final path of the implant. The Nexplanon is inserted using sterile technique. The area is cleaned aseptically, and a 1-percent lidocaine anesthetic track is injected beneath the skin along the planned insertion path. The implant is then placed as shown in Figure 38-4. After placement, both patient and provider should palpate and identify both ends of the 4-cm implant. To minimize bruising at the site, a pressure bandage is created around the arm and is removed the following day.
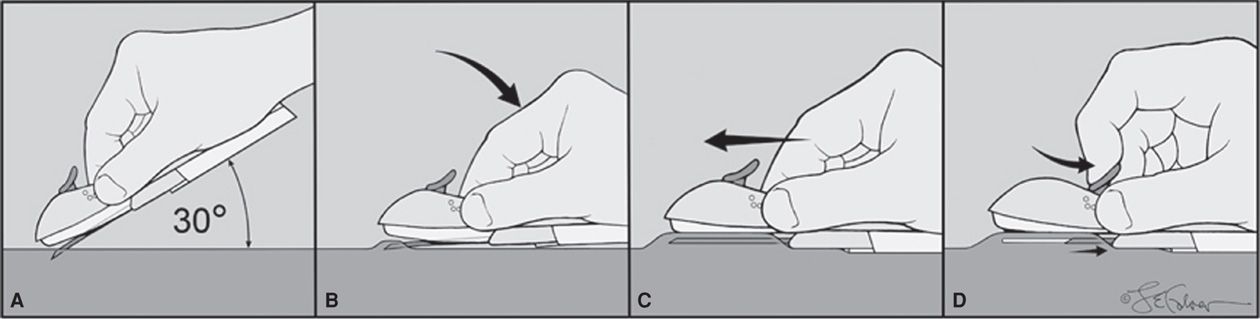
FIGURE 38-4 Nexplanon insertion. A sterile pen marks the insertion site, which is 8 to 10 cm proximal to the medial humeral condyle. A second mark is placed 4 cm proximally along the arm’s long axis. The area is cleaned aseptically, and a 1-percent lidocaine anesthetic track is injected along the planned insertion path. A. The insertion device is grasped at its gripper bubbles found on either side, and the needle cap is removed outward. The device can be seen within the needle bore. The needle bevel then pierces the skin at a 30-degree angle. B. Once the complete bevel is subcutaneous, the needle is quickly angled downward to lie horizontally. C. Importantly, the skin is tented upward by the needle as the needle is slowly advanced horizontally and subdermally. D. Once the needle is completely inserted, the lever on the top of the device is pulled backward toward the operator. This retracts the needle and thereby deposits the implant. The device is then lifted away from the skin. After placement, both patient and operator should palpate the 4-cm implant.
With sterile implant removal, the proximal end of the implant is depressed with a finger to allow the distal end to bulge toward the skin. After anesthetizing the skin over this bulge, the skin is incised 2 mm toward the elbow along the long axis of the arm. The proximal butt of the implant is then pushed toward this incision. Once visible, the distal implant is grasped with a hemostat and removed. If present, superficial adhesions surrounding an implant may be dissected away with hemostat tips placed into the incision.
PROGESTIN-ONLY CONTRACEPTIVE GROUP
 Actions and Side Effects
Actions and Side Effects
Progestin-only contraceptives include the implants just described, pills, and injectables. As their primary contraceptive action, these progestins suppress luteinizing hormone (LH) and in turn block ovulation. As other effects, cervical mucus is thickened to retard sperm passage, and atrophy renders the endometrium unfavorable for implantation. Fertility is restored rapidly following cessation of progestin-only contraception, with the exception of DMPA as described on page 711 (Mansour, 2011).
For all progestin-only methods, irregular uterine bleeding is a distinct disadvantage. It may manifest as metrorrhagia or menorrhagia and is the most frequently reported adverse event leading to method discontinuation. Often, counseling and reassurance regarding this effect is sufficient. Particularly troublesome bleeding may be improved by one to two cycles of COCs, by a 1- to 3-week course of estrogen alone, or by a short course of NSAIDs combined with the established method. Fortunately, with prolonged use, progestins induce endometrial atrophy, which leads to sustained amenorrhea. And, for the well-counseled patient, this is often an advantage.
Most progestin-only contraceptive methods do not significantly affect lipid metabolism, glucose levels, hemostatic factors, liver function, thyroid function, and blood pressure (Dorflinger, 2002). Moreover, these have not been shown to increase the risk for thromboembolism, stroke, or cardiovascular disease (Mantha, 2012; World Health Organization, 1998). However, as described on page 711, the increased low-density lipoprotein (LDL) cholesterol and decreased high-density lipoprotein (HDL) cholesterol levels seen with DMPA may be less desirable if there are cardiac or vascular risks.
Progestin-only methods do not impair milk production and are an excellent choice for lactating women. There are no increased risks of genital tract or breast neoplasia (Wilailak, 2012; World Health Organization, 1991a,b, 1992). Weight gain and bone mineral density loss are not prominent side effects of this contraceptive group, except for DMPA, as noted on page 711 (Funk, 2005; Lopez, 2011). Functional ovarian cysts develop with a greater frequency in women using progestin-only agents, although they do not usually necessitate intervention (Brache, 2002; European Society of Human Reproduction and Embryology, 2001). Last, an association between depression and DMPA or POPs is unclear (Civic, 2000; Svendal, 2012; Westhoff, 1995). Women with depression may be prescribed these methods, but surveillance following initiation is reasonable.
 Contraindications to Progestin-Only Contraceptives
Contraindications to Progestin-Only Contraceptives
These methods are ideal for most women, but contraindications and cautions are associated with a few conditions. Current breast cancer and pregnancy are the only two absolute contraindications. However, conditions for which limited and cautious use should be considered are listed in Table 38-3.
There are a few instances in which manufacturer restrictions differ from the US MEC. First, manufacturer prescribing information lists thrombosis or thromboembolic disorders as contraindications (Merck, 2012a; Pfizer, 2012). However, for individuals with these disorders, US MEC considers progestin-containing methods category 2. Second, for many progestin products, manufacturers note prior ectopic pregnancy as a contraindication. This is secondary to progesterone’s effect to slow fallopian tube motility and thereby delay fertilized egg transport to the endometrial cavity. But again, US MEC considers progestin injectables and implants category 1, and progestin-only pills are category 2 for those with prior ectopic pregnancy.
VERY EFFECTIVE CONTRACEPTION: HORMONAL CONTRACEPTIVES
These currently are available in forms that contain both estrogen and progestin or contain only progestin. Progestin-only injectables and pills are considered very effective, yet second-tier agents, due to the need for increased patient compliance. Similarly, products containing both estrogen and progestin, often termed combination hormonal contraception (CHC), are considered in this tier. These may be supplied as pills, transvaginal rings, or transdermal patches.
 Combination Hormonal Contraceptives Mechanism of Action
Combination Hormonal Contraceptives Mechanism of Action
The contraceptive actions of CHCs are multiple, but the most important effect is suppression of hypothalamic gonadotropin-releasing factors. This in turn blocks pituitary secretion of follicle-stimulating hormone (FSH) and LH to inhibit ovulation. The progestin component provides ovulation prevention by suppressing LH; they thicken cervical mucus and thereby retard sperm passage; and they render the endometrium unfavorable for implantation. Estrogen blocks ovulation by suppressing FSH release. It also stabilizes the endometrium, which prevents intermenstrual bleeding—also known as breakthrough bleeding. The net effect is an extremely effective yet highly reversible method (Mansour, 2011).
 Combination Oral Contraceptive Pills
Combination Oral Contraceptive Pills
Composition
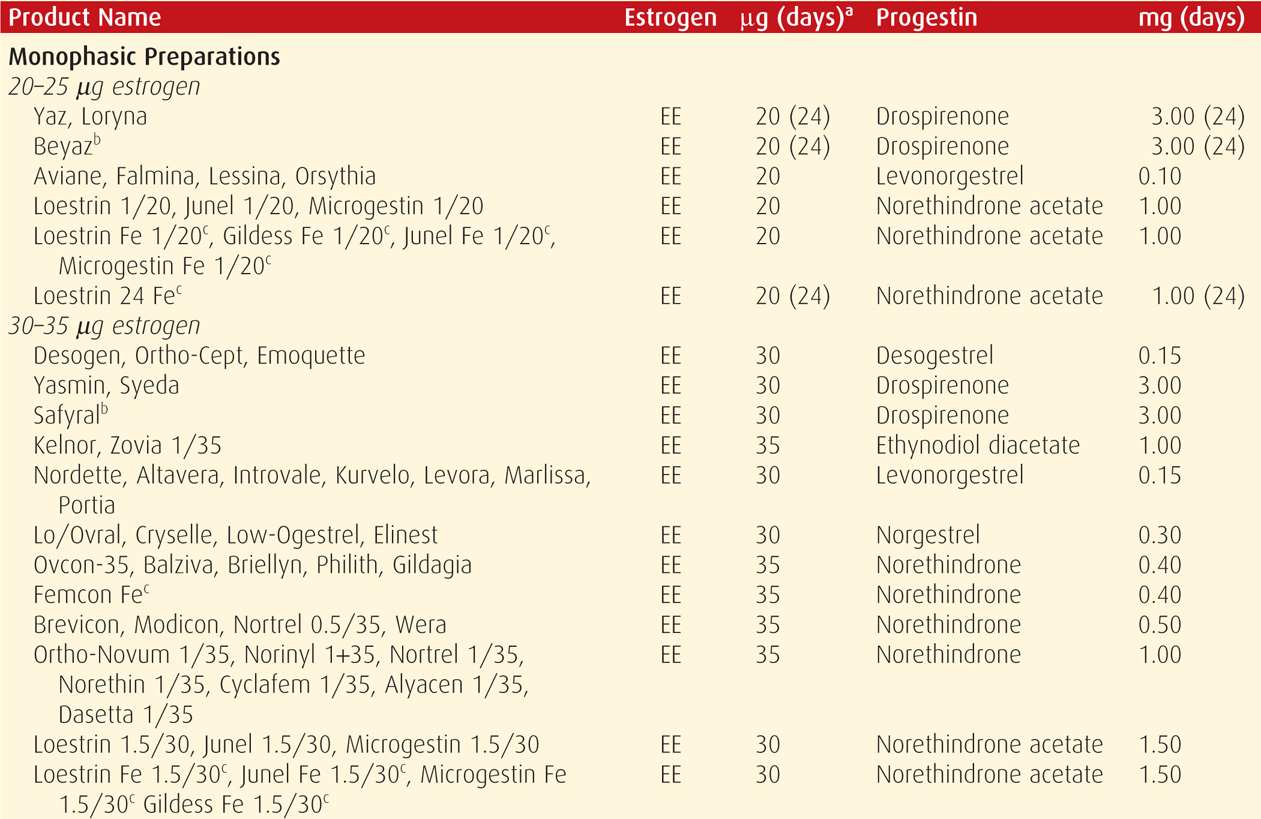
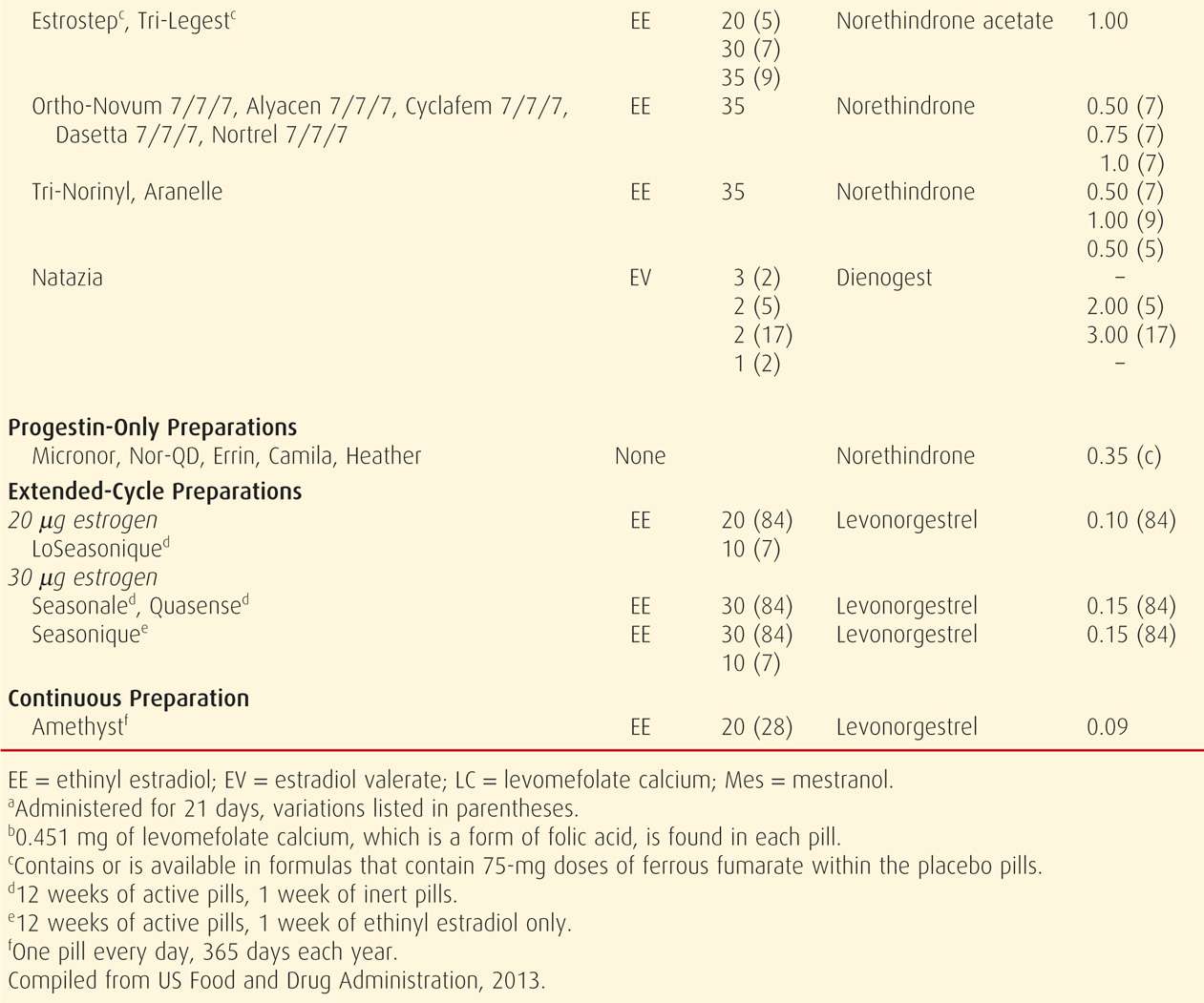
Combination oral contraceptive pills are the most frequently used birth control method in the United States. In a 2006 to 2010 survey, 17 percent of US women were using these (Jones, 2012). COCs are marketed in an almost bewildering variety (Table 38-4). Most are also available as generics, and the FDA (2013) confirms the bioequivalence of COC generics. The American College of Obstetricians and Gynecologists (2013b) supports the use of either branded or generic preparations.
Pharmacologically, ethinyl estradiol is the most common estrogen present in COC formulations in the United States. Less frequently, mestranol or estradiol valerate is used. Unwanted effects most often attributed to the estrogen component include breast tenderness, fluid retention, weight gain, nausea, and headache.
COCs also contain one of several progestins that are structurally related to progesterone, testosterone, or spironolactone. Thus, these progestins bind variably to progesterone, androgen, estrogen, glucocorticoid, and mineralocorticoid receptors. These affinities explain many pill-related side effects and are often used to compare one progestin with another.
Most progestins used in COCs are related to testosterone and may impart androgenic side affects such as acne and adverse HDL and LDL levels. To avoid these effects, progestins more structurally similar to the progestone molecule have been developed. Medroxyprogesterone acetate is one example, but it is mainly used in a progestin-only injectable form. Another, nomegestrol acetate, is used in a COC approved outside the United States. Despite these pharmacological differences, the true advantage of one progestin over another is less apparent clinically (Lawrie, 2011; Moreau, 2007).
Another progestin, drospirenone, is structurally similar to spironolactone. The doses used in currently marketed COCs have effects similar to 25 mg of this diuretic hormone (Seeger, 2007). Drospirenone displays antiandrogenic activity, provides an antialdosterone action to minimize water retention, and has antimineralocorticoid properties that may, in theory, cause potassium retention and hyperkalemia (Krattenmacher, 2000). Thus, it is avoided in women with renal or adrenal insufficiency or with hepatic dysfunction. Moreover, serum potassium level monitoring is recommended in the first month for patients chronically treated concomitantly with any drug associated with potassium retention. These include NSAIDs, angiotensin-converting enzyme (ACE) inhibitors, angiotensin II antagonists, heparin, aldosterone antagonists, and potassium-sparing diuretics (Bayer HealthCare Pharmaceuticals, 2012).
Since the development of COCs, their estrogen and progestin content has dropped remarkably to minimize adverse effects. Currently, the lowest acceptable dose is limited by the ability to prevent pregnancy and to avoid unacceptable breakthrough bleeding. Thus, the daily estrogen content varies from 10 to 50 μg of ethinyl estradiol, and most contain 35 μg or less.
With COCs termed monophasic pills, the progestin dose remains constant throughout the cycle. In others, the dose frequently is varied, and terms biphasic, triphasic, or quadriphasic pill are used depending on the number of dose changes within the cycle. In some formulations, the estrogen dose also varies. In general, phasic pills were developed to reduce the total progestin content per cycle without sacrificing contraceptive efficacy or cycle control. The theoretical advantage of a lower total progesterone dose per cycle has not been borne out clinically (Moreau, 2007). Cycle control also appears to be comparable among mono- through triphasic pills (van Vliet, 2006, 2011a,b).
Administration
Hormones are taken daily for a specified time (21 to 81 days) and then replaced by placebo for a specified time (4 to 7 days), which is called the “pill-free interval.” During these pill-free days, withdrawal bleeding is expected.
With the trend to lower estrogen doses to minimize side effects, there is concern for follicular development and ovulation. To counter this, the active-pill duration in some formulations is extended to 24 days. And, these 24/4 regimens do appear to reduce ovulation and breakthrough bleeding rates (Fels, 2013).
Alternatively, longer durations of active hormone, designed to minimize the number of withdrawal episodes, have been introduced (Edelman, 2006). These extended-cycle products produce a 13-week cycle, that is, 12 weeks of hormone use, followed by a week for withdrawal menses. The product Amethyst provides continuous active hormone pills for 365 days each year. Such extended or continuous regimens may be especially suited for women with significant menstrual symptoms.
For general initiation, women should ideally begin COCs on the first day of a menstrual cycle. In such cases, a supplementary contraceptive method is unnecessary. With the more traditional “Sunday start,” women begin pills on the first Sunday that follows menses onset, and an additional method is needed for 1 week to prevent conception. If menses begin on a Sunday, then pills are begun that day and no supplementary method is required. Alternatively, with the “Quick Start” method, COCs are started on any day, commonly the day prescribed, regardless of cycle timing. An additional method is used during the first week. This latter approach improves short-term compliance (Westhoff, 2002, 2007b). If the woman is already pregnant during Quick Start initiation, COCs are not teratogenic (Lammer, 1986; Rothman, 1978; Savolainen, 1981). Similarly, same-day initiation can be implemented with the contraceptive vaginal ring or patch (Murthy, 2005; Schafer, 2006).
For maximum efficiency, pills should be taken at the same time each day. If one dose is missed, contraception is likely not diminished with higher-dose monophasic COCs. Doubling the next dose will minimize breakthrough bleeding and maintain the pill schedule. If several doses are missed or if lower-dose pills are used, the pill may be stopped, and an effective barrier technique used until menses. The pill may then be restarted. Alternatively, a new pack can be started immediately following identification of missed pills, and a barrier method used for 1 week. If there is no withdrawal bleeding, the woman should continue her pills but seek attention to exclude pregnancy.
With initiation of COCs, spotting or bleeding is common. It does not reflect contraceptive failure and typically resolves within one to three cycles. If unscheduled bleeding persists, those with bleeding during the first part of a pill pack may benefit from an increase in the pill’s estrogen dose, whereas those with bleeding during the second part may improve with a higher progestin dose (Nelson, 2011).
 Method-Specific Effects
Method-Specific Effects
Altered Drug Efficacy
Combination oral contraceptives interfere with the actions of some drugs. In such cases, doses can be adjusted as shown in Table 38-5. Conversely, some drugs decrease the COC effectiveness. Three groups are notable: the antitubercular drugs rifampin and rifabutin; efavirenz and ritonavir-boosted protease inhibitors, which are used to treat HIV infection; and enzyme-inducing anticonvulsants, which include phenytoin (Dilantin), carbamazepine (Tegretol), oxcarbazepine (Trileptal), barbiturates, primidone (Mysoline), and topiramate (Topamax) (Gaffield, 2011; Panel on Antiretroviral Guidelines for Adults and Adolescents, 2013). With these, a method other than COCs is preferable. However, if a COC is selected for concurrent use with an agent from these three groups, then a preparation containing a minimum of 30 μg ethinyl estradiol should be chosen.
TABLE 38-5. Drugs Whose Effectiveness Is Influenced by Combination Oral Contraceptives
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree