Female menstrual cycle
Hormonal abnormalities increase as renal function progressively worsens [2]. With decreased renal clearance, prolactin levels increase which in turn suppresses the release of sex hormones [3]. In addition, the pulsatile release of GnRH and LH is lost, thus leading to anovulation. An increased level of prolactin (PRL) in this population also contributes to the disruption of the hypothalamus-pituitary-gonadal axis. PRL levels are increased due to both decreased renal clearance and impaired inhibition by dopamine [4]. This can occur both in women who are on peritoneal dialysis (PD) or hemodialysis (HD). When women with chronic kidney disease (CKD) are treated with estrogen agonist/antagonist clomiphene citrate, the pulsatile release of GnRH can be restored, thus leading to ovulation [4]. Despite the capability of ovulation, pregnancy in this population can be high risk.
Conception can also be compromised by poor libido and impaired sexual function. Uremia, vasculopathy, neuropathy, comorbid conditions, and medication side effects can all contribute to this [2]. Anti-müllerian hormone (AMH) is a hormone secreted to stimulate follicular growth in the ovaries and has been used as a measurement of ovarian reserve. In a study of women with CKD and regular menses, the levels of AMH are lower than that seen in controls [5]. Authors of this study have suggested that uremia may lead to destruction of ovarian cells, thus reducing levels of AMH. Also the amenorrhea seen in ESRD can lead to vaginal dryness and decreased libido [1]. Vaginal lubrication or estrogen therapy may ameliorate this problem, as well as renal transplantation [6]. Sexual desire can be an issue for women with chronic kidney disease prior to the need for HD or PD due to uremia. This may improve after the onset of dialysis or after a renal transplant [7]. In the case of PD, the PD catheter itself is in the lower abdomen, which can lead to body image issues [8].
The Physiology of Insulin and Glucose Regulation
The endocrine pancreas consists of the islets of Langerhans which contain many cells, including alpha and beta cells. Alpha cells secrete glucagon to increase plasma glucose levels, and beta cells secrete insulin to increase the rate of glucose transport into striated muscle cells and adipocytes [9]. In muscle cells, glucose is then either stored as glycogen or oxidized to generate adenosine triphosphate for energy [9]. In adipose tissue, glucose is mostly stored as lipid. Besides promoting lipid synthesis, insulin also inhibits lipid degradation in adipocytes [9]. Similarly, insulin promotes amino acid uptake and protein synthesis while inhibiting protein degradation [9]. Thus, the metabolic effects of insulin can be summarized as anabolic, with increased synthesis and reduced degradation of glycogen, lipid, and protein [9]. Insulin and glucagon have opposing regulatory effects on glucose homeostasis . Normal glucose homeostasis is tightly regulated by three interrelated processes that include glucose production in the liver, glucose uptake and utilization by peripheral tissues, chiefly skeletal muscle, and actions of insulin and counterregulatory hormones (e.g., glucagon) [10].
In a person without diabetes, insulin is released in a pulsatile and rhythmic fashion with approximately one unit released per hour. There are two identifiable basal rhythms released every 5–10 minutes and 60–120 minutes [11]. There is also a diurnal pattern with peak secretion in the waking hours of the morning and lowest in the middle of the night [11]. There is an additional large secretory burst of insulin released following a meal in response to high levels of ingested glucose [11]. In the fasting state, insulin levels are low and glucagon levels rise facilitating hepatic glucose synthesis and glycogen degradation for utilization [10]. Following a meal, glucagon levels fall and insulin levels rise in response to the ingested glucose load [10]. In peripheral tissues, insulin binds to the insulin receptor triggering numerous intracellular responses that promote glucose uptake and postprandial glucose utilization, thereby maintaining glucose homeostasis [9]. Abnormalities at various points along this complex signaling cascade, from synthesis and release of insulin to insulin receptor interactions in peripheral tissues, can result in the diabetic phenotype [12]. The two most common phenotypes are type 1 diabetes mellitus and type 2 diabetes mellitus.
Type 1 diabetes mellitus (T1DM) is an autoimmune disease (in more than 95% of cases) in which beta cell destruction is primarily caused by immune effector cells [13]. Patients with T1DM have insulin deficiency and are dependent on exogenous insulin to sustain life [13]. If untreated, T1DM is a fatal catabolic disorder [10]. T1DM management includes insulin therapy that mimics normal insulin physiology with a basal long-acting insulin (lasts for up to 24 hours) and a pulse of short-acting insulin for the estimated mealtime glucose load. Over time, patients may learn to count carbohydrates to estimate a near accurate mealtime insulin dose based on their food choices.
There are other insulin formulations such as intermediate acting insulin, short acting insulin, or premixed insulins that may be preferred as a low-cost insulin option. These may, however, portend a slightly higher risk of hypoglycemia as compared to the long-acting and rapid-acting insulins [14]. Newer technological advances such as the continuous glucose monitoring (CGM) system which is a subcutaneous (subQ) device that provides real-time interstitial fluid glucose concentration and alarms the patient if blood glucose is dangerously high or low. The use of this device is particularly helpful in patients with brittle diabetes [15]. Finally, insulin can also be delivered via a continuous subcutaneous insulin infusion (CSII) device. This device usually has two components: (1) a subcutaneous port for insulin delivery in the body and (2) a controller that transmits insulin dosing instructions to the subQ port for insulin delivery [16]. This system provides flexibility and is optimal for patients who prefer not to take multiple daily injections (MDI).
Type 2 diabetes mellitus (T2DM) is a complex multifactorial disorder of blood glucose regulation. The defects that characterize type 2 diabetes include a decreased ability of peripheral tissues to respond to insulin (insulin resistance) and beta cell dysfunction that is manifested as inadequate insulin secretion in the face of insulin resistance and hyperglycemia [17]. More than 50% of beta cells are already impaired by the time T2DM is diagnosed due to increased beta cell apoptosis and decreased beta cell mass [18]. T2DM is related to polygenic factors including abdominal visceral obesity, sedentary lifestyle, aging, and genetic predisposition with a strong underlying family history [10]. Multiple approaches can be used for T2DM management including aggressive lifestyle modification in addition to either oral agents, injectable therapy, or exogenous insulin. Sometimes, concentrated insulin formulations (Humulin® U500 insulin) and/or CSII devices are also used in patients with T2DM based on insulin requirements. Rarely, CGM is used if the patient has frequent hypoglycemia or noted to have brittle diabetes.
Diabetes Mellitus and End-Stage Renal Disease
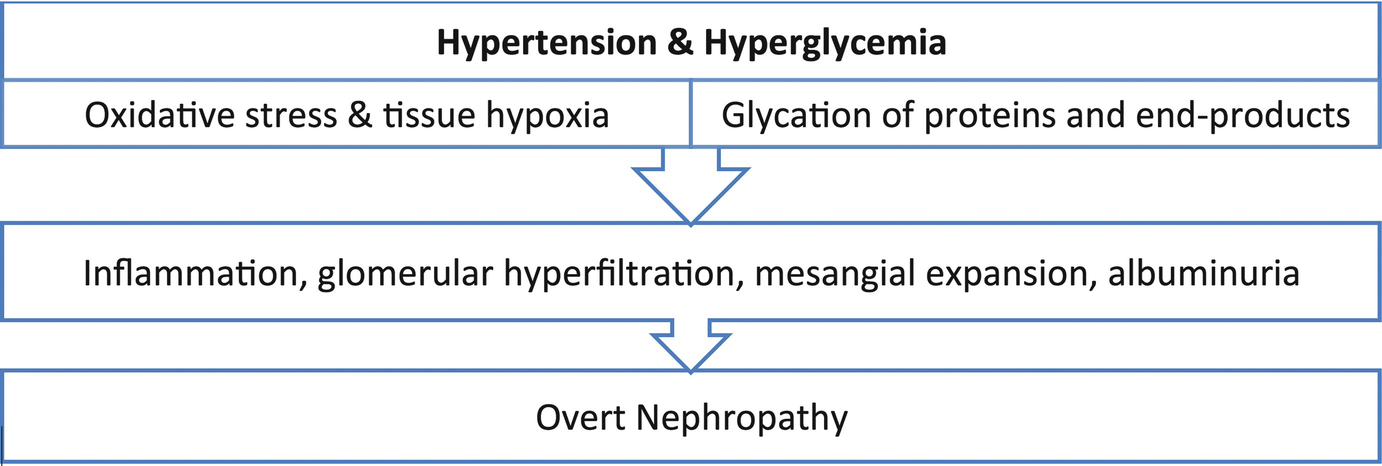
Uncontrolled hypertension and hyperglycemia resulting in overt nephropathy
The mainstay of treatment of diabetic nephropathy is optimizing glycemic control and hypertension management [21]. Many patients have associated anemia from CKD and anemia of pregnancy that may limit interpretation of glycosylated hemoglobin A1c (HbA1c) as a marker of blood glucose control. HbA1c must be correlated with self-monitored blood glucose readings in patients with any degree of anemia for optimum assessment. Additionally, hypertension management is an integral process in managing diabetic nephropathy [21]. Angiotensin-converting enzyme inhibitors (ACEi) and angiotensin receptor blockers (ARBs) are the antihypertensive drug classes of choice in the nonpregnant patient because these agents appear to have reno-protective effects that exceed their antihypertensive effects [21]. However, ACEi and ARBs are not recommended during pregnancy due to their teratogenic side effects. Calcium channel blockers, beta-blockers (labetalol), and vasodilators (hydralazine) are however considered safe during pregnancy.
Goal of management in pregnant and nonpregnant patients with T1DM or T2DM includes optimizing blood glucose levels and controlling hypertension to prevent the onset (or limit worsening) of microalbuminuria and progressive renal dysfunction [22]. Many oral antidiabetic agents are dose adjusted for the degree of CKD stage in nonpregnant T2DM patients. Metformin and the sulfonylurea glyburide are the only two oral agents that are used in pregnancy for gestational diabetes mellitus (GDM) or T2DM; however, metformin is not initiated in patients with CKD stage 3 or higher due to the risk of metformin-associated lactic acidosis (MALA) [21]. Sulfonylureas as a class are notoriously known to cause hypoglycemia in all patients but significantly higher risk is noted in patients with CKD or ESRD [21]. Insulin must be dosed cautiously for all patients with underlying renal impairment or on dialysis to prevent the adverse effects of hypoglycemia.
Diabetes Management in Pregnant Patients with Chronic Kidney Disease or ESRD
Early pregnancy is a time of insulin sensitivity, lower glucose levels, and lower insulin requirements in women with type 1 diabetes [23]. The situation rapidly reverses as insulin resistance increases exponentially during the second and early third trimesters and levels off toward the end of the third trimester [23]. In women with normal pancreatic function, insulin production is sufficient to meet the challenge of this physiological insulin resistance and to maintain normal glucose levels [9]. However, patients at risk for T2DM (personal history of GDM, prediabetes, acanthosis nigricans, obesity, family history of T2DM) are usually screened prior to planned pregnancy (with HbA1c) or in the first trimester of pregnancy (with oral glucose tolerance test, OGTT) for diagnosis of overt T2DM [24]. All pregnant patients are usually screened for GDM between weeks 24 and 28 of pregnancy with OGTT [24]. A positive test result in a pregnant patient with no previous history of diabetes mellitus is diagnostic of GDM, defined as hyperglycemia or glucose intolerance with an onset or first recognition during pregnancy.
Diabetes management during pregnancy alone can be difficult and even more challenging in patients with underlying moderate to severe chronic kidney disease. CKD is related to a high risk of both maternal renal failure and perinatal mortality [25]. It can increase the risk of hypertension, preterm delivery, preeclampsia, intrauterine growth restriction (IUGR), neonatal jaundice, and mechanical ventilation of the infant. In women with elevated serum creatinine 1.4–2.7 mg/dl, 43% had persistent loss of renal function at 6 months postpartum (the same CKD stage as pre-pregnancy). Fifty-nine percent of these pregnancies were complicated by preterm delivery, and 37% had IUGR [26]. In a case series, pregnant patients with diabetic nephropathy and moderate to severe renal insufficiency were found to have a greater than 40% chance of accelerated progression of their renal disease because of pregnancy with many requiring hemodialysis in the postpartum setting [27]. Additionally, infants born from mothers with diabetic nephropathy and poorly controlled diabetes have an increased burden of morbidity. In a 1 year study of infants of 36 women with diabetic nephropathy followed postpartum, 1 child suddenly died 29 days following delivery, 4 children had severe psychomotor retardation, and 1 had severe motor retardation [25]. The risk of prematurity (delivered prior to 34 weeks gestation) and perinatal morbidity was significantly higher in these infants [25].
Management of pregnant patients with diabetes and chronic kidney disease (all stages) or ESRD begins with preconceptual counselling, aggressive hypertension and hyperglycemia management, routine close follow-up visits with maternal-fetal medicine (MFM) physicians from the beginning of pregnancy, and adhering to recommendations throughout pregnancy. A goal HbA1c <7% or as close to 6% with minimal hypoglycemia and well-controlled blood pressure (<140/90) is recommended prior to pregnancy in patients with both type 1 and type 2 diabetes [28]. Pregnancy worsens retinopathy and nephropathy, and patents are strongly advised to complete retinopathy screening prior to pregnancy, during pregnancy, and 1 year postpartum due to risk of worsening retinopathy and macular edema [28]. A thorough review of medications must be undertaken to eliminate medications with potential harm to the fetus, such as statins, ACEi, or ARBs that are commonly prescribed in patients with diabetes mellitus.
Blood glucose targets for patients with T1DM, T2DM, or GDM during pregnancy with or without ESRD
Blood glucose targets for patients with T1DM or T2DM or GDM during pregnancy |
Fasting blood glucose 60–95 mg/dL and either: |
1 hour postprandial blood glucose <140 mg/dL |
2 hour postprandial blood glucose <120 mg/dL |
Medical nutrition therapy (MNT) is recommended for diabetes management with a focus on consistent timing, quality of healthy foods, and carbohydrate counting. Prenatal vitamins with folic acid reduce the risk of congenital malformations in infants [29]. Metformin is pregnancy category B, and glyburide is pregnancy category C; they are cautiously prescribed in patients with CKD due to risk of lactic acidosis and hypoglycemia, respectively [29]. Also both drugs cross the placenta. The National Institute for Health (NIH) recommends cautionary use of metformin when serum creatinine exceeds 130 μmol/L (1.5 mg/dL) or an estimated glomerular filtration rate (eGFR) falls below 45 mL/min per 1.73 m2. The NIH further specifies that metformin be stopped if serum creatinine exceeds 150 μmol/L (1.7 mg/dL) or eGFR is below 30 mL/min per 1.73 m2 [30, 31]. Insulin generally does not cross the placenta and is considered safe in pregnancy with some exceptions. Long-term risks of insulin glargine® during pregnancy are not known; thus, glargine® is pregnancy category C per the Food and Drug Administration (FDA) [29]. Detemir® was found non-inferior to neutral protamine Hagedorn (NPH) insulin and is considered pregnancy category B. For rapid-acting insulin, both aspart® and lispro® are considered safe during pregnancy [29]. Newer insulin analogues are not well studied for pregnant populations.
CGM devices are highly beneficial as they provide real-time feedback to alter diabetes management for optimal blood glucose control in this patient population. MDI may be cumbersome in pregnant patients with CKD or ESRD, and any improper dosing may result in dangerous hypoglycemia. CSII therapy (with or without CGM) may be safer in these patients to optimize blood glucose management while minimizing hyper- or hypoglycemia. In pregnancy, superiority of CSII over MDI has not been demonstrated due to lack of studies.
Thyroid Hormone Production and Metabolism
Thyroid hormone production begins in the hypothalamus with thyrotropin-releasing hormone (TRH) which stimulates the pituitary to release thyrotropin-stimulating hormone (TSH). TSH then stimulates the thyroid to make thyroid hormones: thyroxine (T4) and triiodothyronine (T3). Most T3 and T4 circulate bound to albumin or transthyretin, which is known as total thyroxine (TT4) or total triiodothyronine (TT3) [32].
Thyroid Physiology in Renal Failure
Renal failure is associated with alterations in thyroid hormone binding and metabolism leading to lower detected levels of free thyroxine (FT4) [33], despite having a normal TSH [3]. FT4 is measured by separating FT4 from albumin or other proteins. In renal failure, organic compounds which are not cleared by dialysis remove albumin from the tracer used to detect T4 [33]. Having fewer bound albumin-tracer sites indicates more TT4 and less FT4, yet both are lower than normal. In states such as nephrotic syndrome or renal failure, the levels of albumin are lower than normal, which also leads to decreased binding of thyroid hormone, thus causing lower levels of FT4 [32].
Thyroid Physiology in Pregnancy
During pregnancy human chorionic gonadotropin (hCG) rises exponentially in the first trimester, with a maximal level at week 10 [33]. It is homologous to TSH; thus it stimulates the TSH receptor of thyroid follicular cells to make more thyroid hormone [34]. The fetal thyroid gland is also stimulated early in pregnancy [35]. By week 12, the fetus is making thyroid hormone on its own [32]. The high levels of hCG in the first trimester also allows for increased stimulation of the mother’s gland which can cause hyperthyroidism: a low TSH with an elevated FT3 and FT4. In addition TT3 and TT4 are increased by an estrogen-stimulated increase in serum thyroid-binding globulin (TBG) [35]. These binding alterations lead to inaccurate measurements of FT3 and FT4 in pregnancy. The use of liquid chromatography-tandem mass spectrometry is the more reliable test in pregnancy [36]. Unfortunately it is not as widely available as the two-step immunoassays. The Endocrine Society instead recommends using the TT4 and readjusting the nonpregnant normal range multiplying it by 1.5 to account for the binding alterations noted above [37]. For some women hyperthyroidism may not simply be physiologic; thus it is important to rule out other causes of hyperthyroidism such as Graves’ disease (the most common cause of hyperthyroidism in pregnancy) or toxic multi-nodular goiter [34]. This requires taking a good history as palpitations, weight loss, fatigue, and heat intolerance are common symptoms [38]. In addition a good physical exam is needed to assess for orbitopathy, goiter, or thyroid nodules, as well as serum testing for antibodies such as thyroid receptor antibody (TRAb) [39].
Other changes during pregnancy are related to thyroid hormone production. Iodine levels are reduced as it is consumed by both the maternal and fetal gland. Increased activity of placental deiodinase D3 also aids in providing iodine to the placenta [35]. In addition there is an increased renal clearance of iodine during pregnancy. As a result the World Health Organization (WHO) recommends that pregnant women take 250 mcg of iodine daily during pregnancy and lactation, whereas the American Thyroid Association suggests 150 mcg/day of iodine supplementation during pregnancy and an additional consumption of 250 mcg of dietary iodine during breastfeeding [40]. This is especially important for those who live in iodine-deficient areas. For those who are iodine deficient, the body compensates by preferentially using T3, to decrease the amount of iodine consumed. Over time the T3 production becomes insufficient, thus increasing the TSH and leading to maternal hypothyroidism, as well hypothyroidism of the fetus because the fetus makes both T3 and T4 [35].
Thyroid Disease Management in Pregnant Women with ESRD
In women who already had a diagnosis of hypothyroidism or hyperthyroidism, medications will need adjustment due to aforementioned physiological changes of pregnancy. In women with a history of hypothyroidism, it is generally observed that there will be at least a 30% increase in thyroid hormone dose during the pregnancy [37]. As a result, it is recommended that a woman takes two extra doses of levothyroxine weekly once pregnancy is confirmed and contacts her endocrinologist [40]. As recommended in all persons with hypothyroidism, levothyroxine should be taken on an empty stomach 60 minutes prior to eating or taking other medications or vitamins to avoid poor absorption. Desiccated thyroid hormone, which has both T4 and T3, should not be used in pregnancy as T3 is unable to cross into the fetal brain [40].
For those with hyperthyroidism, achieving euthyroidism is important to avoid miscarriage, congestive heart failure, or even fetal death [39]. Methimazole (MMI) and propylthiouracil (PTU) are antithyroid agents used to decrease thyroid hormone production. PTU is preferred in the first trimester, as MMI is associated with the scalp deformity aplasia cutis congenita if used during that time [34]. PTU is associated with direct hepatotoxicity; thus it has become a second-line agent with the exception of the first trimester of pregnancy and allergy/intolerance of MMI. Both can be used during breastfeeding. Beta-blocker therapy can also be used for symptoms of hyperthyroidism. In pregnancy, labetalol is most commonly used [41]. In breastfeeding metoprolol or propranolol can be prescribed. If medications are ineffective due to adverse effects or poorly controlled disease, thyroidectomy in the second trimester is an alternative option. However, in the case of autoimmune disease, the antibodies can remain present and still stimulate fetal hyperthyroidism. Radioactive iodine with I131 to shrink the gland by cellular destruction is an option of management, but it’s contraindicated in pregnancy and for up to 6 weeks after cessation of breastfeeding [38].
In those without an established diagnosis of thyroid disease, universal screening is not recommended; however, women who are high risk should be screened. Also the TSH should ideally be 2.5 mIU/L or less in those who are in the preconception phase [37]. High-risk factors include age over 30 years, history of thyroid disease, history of head or neck irradiation, history of thyroid surgery, family history of thyroid disease, typical symptoms, goiter, presence of thyroid peroxidase antibody (TPO Ab), history of miscarriage or preterm delivery, or those who live in an iodine-deficient area [42].
Parathyroid Hormone Production and Metabolism
The calcium-sensing receptor (CaSR) on parathyroid cells regulates the release of PTH [43]. Hyperparathyroidism can be either primary or secondary. Primary hyperparathyroidism is typically due to parathyroid adenoma. Secondary hyperparathyroidism is often the result of vitamin D deficiency or renal disease. The converse, secondary hypoparathyroidism is typically due to a thyroidectomy. In such cases, vitamin D and calcium replacement are important to avoid hypocalcemia, which can lead to tetany and preterm labor [44].
Parathyroid Hormone and Renal Failure
Vitamin D is mainly absorbed in the skin from the sun but can also be found in the diet. It is then hydroxylated to vitamin D 25-OH (25(OH)D) by the liver. It is then converted to the active form of vitamin D: vitamin D 1,25-OH (1,25 (OH)2D) in the kidneys by 1α-hydroxylase. In persons with renal failure, this process does not occur for multiple reasons. For those who are on peritoneal dialysis (PD), 25(OH)D is removed before the second hydroxylation can occur [3]. Also the activity of 1α-hydroxylase decreases as renal function worsens. Lastly, as renal function declines, there is an increase in fibroblast growth factor 23 (FGF23), which also inhibits 1α-hydroxylase. The low level of active vitamin D results in decreased gut absorption of calcium, thus stimulating PTH release. As PTH levels rise, hyperplasia of parathyroid cells can develop leading to parathyroid hyperplasia which if left untreated can lead to tertiary hyperparathyroidism [43].
Parathyroid Physiology in Pregnancy
Parathyroid hormone is secreted by maternal parathyroid glands. Hydroxylation of 25(OH)D is completed by the maternal kidneys and the placenta. The placental hydroxylation to calcitriol is dependent on maternal 25(OH)D stores [45]. The rise in calcitriol is not dependent on PTH levels. In a study of PTH-null mice, their levels of calcitriol rose fivefold as seen in wild-type mice, suggesting that PTH is not required for this surge in calcitriol seen during pregnancy [46]. In a study of calcitriol levels in persons with PHPT, pregnancy, renal failure, and healthy controls, not surprisingly those with pregnancy and PHPT had the highest calcitriol levels [47]. Independent of renal disease, women with secondary hyperparathyroidism (SHPT) have an increased risk of preeclampsia compared to those with low 25(OH)D and a normal PTH [48].
Management of Parathyroid Disease in Pregnant Women with ESRD
Calcium, phosphorus, and parathyroid levels should be routinely monitored in the pregnant woman on dialysis. Adjustment of dialysate calcium bath and the use of vitamin D analogues or phosphate binders are dictated by the patient’s clinical situation. Of note, the phosphorus binder Sevelamer® has been associated with bone deformities in rodents and should be used with caution [49]. In addition, there is limited data on the use of the calcimimetic Cinacalcet® during pregnancy [50, 51].

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


