Vascular tumor
Grows by endothelial proliferation (“-oma”)
May involute
Vascular malformation
Quiescent, non-proliferating endothelial cells
Never involute
A vascular tumor grows by endothelial proliferation, hence the suffix “-oma.” In contrast, a vascular malformation is composed of nonproliferating endothelial cells. This quiescent but abnormal endothelium is caused by a defect in morphogenesis. Tumors like hemangiomas can involute and are generally treated conservatively. Malformations never involute and instead grow commensurately with the patient. Nevertheless, endothelial proliferation can occur in certain vascular malformations in response to clot formation, increased flow, hormonal changes, and endovascular and surgical intervention. Malformations can be managed by observation, sclerotherapy, embolization, or surgical resection. Treatment is always individualized, but the surgical resection should adhere to the basic principles outlined in this chapter.
The binary schema of vascular tumors and malformations based on biologic activity was accepted by the International Society for the Study of Vascular Anomalies (ISSVA) in 1996 and has been refined and expanded since then with the current classification listed in Table 2. Our understanding of vascular anomalies has grown tremendously through observation, radiographic evaluation, immunohistochemical staining, medical management, surgical triumphs, and complications. Nonetheless, our understanding will continue to evolve with each generation of researchers and surgeons.
Table 2
International Society for the Study of Vascular Anomalies (ISSVA) classification of vascular tumors and malformations
Vascular tumors | Vascular malformations |
|---|---|
Hemangioma | Slow-flow |
Infantile hemangioma (IH) | Capillary malformations |
Congenital hemangioma (CH) | Port-wine stain |
Noninvoluting (NICH) | Telangectasias |
Rapidly involuting (RICH) | Angiokeratoma |
Hemangiomatosis | Venous malformations |
Tufted Angioma (with or without Kasabach–Merritt syndrome) | Common sporadic VM |
Blue rubber-bleb syndrome (BRBS) or Bean syndrome | |
Dermatologic acquired vascular tumors | |
Glomuvenous malformation (GVM) | |
Pyogenic Granuloma | |
Kaposiform hemangioendothelioma (KHE) | Familial cutaneous and mucosal venous malformation (VMCM) |
Spindle-cell hemangioendothelioma | Maffucci syndrome |
Rare tumors | Diffuse VM (Bockenheimer) |
Hemangioendothelioma | Lymphatic malformations (LM) |
Infantile fibrosarcoma | Common lymphatic malformation (LM) |
Hemangiopericytoma | Lymphangiomatosis |
Giant cell angioblastoma | Gorham–Stout syndrome |
Fast-flow | |
Arterial malformation (AM) | |
Arteriovenous malformation (AVM) | |
Arteriovenous fistula (AVF) | |
Complex-combined vascular malformations | |
CVM, CLM, LVM, CLVM (Klippel–Trenaunay) | |
CAVM (Parkes Weber), AVM-LM, CM-AVM | |
Proteus | |
Capillary-lymphatic overgrowth–vascular–epidermal nevus (CLOVE) |
Diagnostic Work-Up
Evaluation begins with a detailed history and physical examination that correctly diagnose the vast majority of vascular anomalies in the upper extremity. It is important to obtain baseline functional data and length and circumference measurements of the limbs early in life and to follow these children as they grow.
The patient with a large, soft, diffuse, asymptomatic lesion may never require investigational studies. Expensive diagnostic studies are similarly unnecessary in the case of a small fast-flow lesion amenable to simple excision and closure. When a patient is symptomatic or if an operation is being considered for a large vascular anomaly, the surgeon should learn as much as possible about the location, size, extent, depth, caliber, and flow characteristics.
The diagnostic work-up varies for each patient and for each type of vascular anomaly. In addition to a comprehensive exam and history, the studies below may be helpful. These studies are also summarized in Table 3.
Table 3
Diagnostic work-up
Hemangioma | CM | VM | LM | Combined | AVM | |
|---|---|---|---|---|---|---|
Physical exam | ++++ | ++++ | ++++ | ++++ | ++++ | ++++ |
Ultrasound | +++ | − | + | + | + | +++ |
MRI, MRV, MRA | ++ | − | +++ | +++ | +++ | +++ |
Radiographs | − | − | ++ (phleboliths, bone) | + (bone) | + (bone) | + (bone) |
CT | + | − | + | + | + | + |
Angiography | − | − | − | − | − | ++ |
Biopsy | + | − | + | + | + | + |
Ultrasonography confirms the initial distinction between a vascular tumor and vascular malformation. A handheld Doppler can easily be used in the clinic to pinpoint the exact location of the lesion and identify it as either slow-flow or fast-flow.
Plain radiology of the affected region provides very little information other than showing the presence of phleboliths and interosseous involvement or other skeletal distortions.
Magnetic resonance imaging (MRI) with gadolinium is the “gold standard” for slow-flow malformations. Fat-suppressed T1-weighted sequences will differentiate a vascular tumor from a malformation. MRI delineates the rheologic characteristics and extent of the malformation and is frequently used as a baseline examination in complex lesions.
Computed tomography (CT) can be used to detect interosseous involvement or skeletal distortion caused by a vascular malformation and can help delineate soft tissue planes. Three-dimensional CT rendering of both fast-flow and slow-flow malformations provides impressive views of the entire lesions and its relationship to neighboring soft tissue and skeletal structures. CT angiography (CTA) cannot substitute for formal angiography in demonstrating all of the smaller, early-filling branches of a fast-flow lesion and does not show the fine detail needed in the hand or brachial plexus.
Angiography is indispensable in the diagnosis, assessment, and characterization of fast-flow lesions but has very little use in the diagnosis of slow-flow malformations. The angio-architecture and three-dimensional anatomy of the malformation can be precisely determined and the arterial suppliers and draining veins clearly identified. Indirect and direct phlebography techniques are sometimes used for diagnostic and therapeutic purposes for a VM in the extremity.
Histopathology can offer a tissue diagnosis to confirm or clarify particularly challenging cases in which physical exam and advanced imaging are not conclusive. Many biologic markers are known for particular lesions and there is a large body of ongoing research attempting to further characterize these anomalies.
Management
Vascular anomaly teams have been assembled in most major referral centers to provide an interdisciplinary approach to diagnosis and management. The initial management is usually nonsurgical. Indeed, surgical resection is considered only after conservative measures have failed to control bulk or symptoms. Consideration must be given to the patient’s age, first appearance of the vascular anomaly, function of the limb, activities of daily living, response to previous treatment, and assessment of the patient’s needs and expectations. The management of these difficult lesions involves a variety of approaches that are compared in Table 4 and described in further detail below.
Table 4
Treatment options of vascular anomalies
Tumors | CM | VM | LM | Combined | AVM | |
|---|---|---|---|---|---|---|
Pharmacologic | +++ | − | − | − | − | + |
Laser | + | +++ | + | + | − | − |
Sclerotherapy | − | − | ++++(axilla, arm) | +++(axilla, arm) | +++ | + |
+++(hand) | ++ (forearm, hand) | |||||
Embolization | ++ | − | + | − | − | ++++ |
Surgical excision | ++++ | − | ++(arm, forearm) | + (axilla) | ++ | ++ |
+++ (arm) | ||||||
+++(digits, thumb) | ||||||
++++(hand) | ||||||
Embolization/excision | +++ | − | − | − | ++ | ++++ |
Nonoperative Management
Compression. Compression garments are the first line of treatment for venous, lymphatic, and mixed malformations and are used to reduce blood stagnation, symptomatic expansion, phlebolith formation, and the chance of localized intralesional coagulopathy (LIC). Children who experience relief of their symptoms with compression can become surprisingly compliant and active in daily life.
Pharmacologic. Pain is often associated with intralesional thrombi or phlebothrombosis of vascular malformations. Daily low-dose aspirin helps to prevent thrombus formation. More definitive anticoagulation and placement of an intraluminal inferior vena caval filter are used selectively in the patient at high risk for thromboembolism. Although long-term anticoagulation intralesional bleeding, with or without nerve compression or muscle compartment swelling, may require appropriate decompression.
Sclerotherapy. Direct-puncture or catheter-introduced sclerotherapy is the next step for venous and lymphatic malformations that cause functional impairment, nerve compression, mass effect, pain, or major contour problems if conservative measures fail. This intervention reduces the size of the malformation, but generates scar and does not remove the malformation. The secondary scar generated by sclerotherapy can be detrimental to nerve function, tendon gliding, and joint motion especially in the distal forearm, hand, and digits. In the upper arm, elbow, and proximal forearm, sclerotherapy is safer and often more effective than resection (Burrows et al. 2004; Smith et al. 2009). Sclerotherapy is preferred for diffuse lesions, except for those with multiple intralesional thromboses, compressive symptoms, or lesions which involve the distal forearm, wrist, hand, and digits. Figure 1 shows the radiographic work-up and injection of a proximal third forearm VM which is ideal for sclerotherapy.
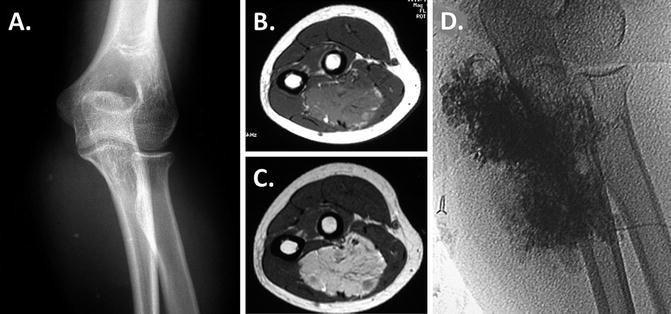

Fig. 1
Sclerotherapy. Radiographic studies of the vulnerable proximal third of the forearm show a VM, which is ideal for sclerotherapy: (a) Shows small calcified phleboliths on radiographs (b). Isointense to adjacent skeletal muscle on axial T1-sequence; (c) signal voids on axial T2 representing phleboliths; (d) lesion fills with direct puncture and injection of contrast
Sclerosant agents include doxycycline, sodium tetradecyl sulfate (STS), ethanol, bleomycin, and OK-432. Ethanol has the highest complication rate; OK-432, although very effective, is not widely available (Burrows et al. 2004). Direct injection of sclerosant near neurovascular structures within the palm of the hand, volar wrist flexion crease, antecubital fossa, and axilla carries a considerable potential risk. The most common complications are skin ulceration (10–15 %), local extravasation, compartment syndrome, and secondary contracture. Multiple sessions are often required.
Surgical Management
Although nonoperative therapy is initially preferred, surgery is mandated when these previous modalities have failed to correct or even control functional loss, compression neuropathies, recurrent infections, and pain. In the adolescent, aesthetics is not an irrelevant consideration.
The advantages of surgical resection include the use of a pneumatic tourniquet, the identification of all anatomic structures, direct removal of the malformation and involved tissues, and sparing of vital structures. Compared to the other modalities, however, surgical resection imparts a longer recovery time with the need for more rigorous rehabilitation. It should be stated from the outset that unless amputated, these lesions can never be absolutely eradicated.
The excision of a vascular malformation is always beset with potential pitfalls, and complications include compartment syndrome, bleeding, wound dehiscence, infection, devascularization of a limb or digit, secondary contracture, tendon adherence, and chronic pain secondary to nerve injury. Adherence to basic surgical principles will minimize these potential problems.
Surgical Principles
1.
Preoperative planning is the cardinal principle of surgical treatment. Most poor outcomes can be avoided by adhering to this principle alone. Planning should include correlation of the size, extent, and involvement of structures with physical examination and imaging. Straying from the plan may lead to a violation of uninvolved areas, an inadvertent injury to neurovascular structures, a waste of precious tourniquet time, and an incomplete excision. Other preparatory steps for complex lesions include central venous and peripheral arterial monitoring, availability of fast-flow warmers and blood products for transfusion, and intensive care unit monitoring postoperatively.
2.
Placement of incisions is important, particularly in children. When planning an incision, surgeons should consider exposure, blood supply, function, aesthetics, and future surgery. In the digits, a high mid-axial incision is preferred because it provides excellent exposure dorsally and volarly and is typically well hidden. Dorsal longitudinal hand and digital incisions are also avoided if possible because they provide less exposure and are more conspicuous. Palmar incisions can lead to contracture if poorly planned; zigzag incisions and those using normal skin creases are recommended. The medial surface of the arm, elbow, and forearm is the least conspicuous.
3.
Dissection in a bloodless field is critical for identification of important structures. Dissection within a heavily bloodstained field portends nerve injury. To this end, the use of the pneumatic tourniquet is essential for all but the simplest excisions. In small children, an Esmarch bandage alone suffices, secured at the mid-humeral level. In upper arm and brachial plexus explorations in which a tourniquet cannot be used, a premium is placed on meticulous hemostasis achieved through careful dissection and preemptive ligation or clipping of vessels. Three types of cautery devices are used selectively. Monopolar cautery is for subcutaneous dissections, bipolar cautery is for small vessels, and the battery-operated ophthalmic cautery is used for developing surgical planes within muscles to avoid fasciculation. Tissue sealants have been a useful adjunct at the end of a procedure once the tourniquet is deflated.
4.
The use of fine surgical tools and magnification is imperative. These complicated dissections cannot be performed without the use of delicate, undamaged, top-quality scissors, fine-toothed forceps, and either loupe or microscope magnification. The identification and preservation of normal neurovascular structures such as vincular pedicles to the flexor tendons and nutrient vessels to the carpal bones is critical. Despite the anatomic distortion, an areolar connective tissue plane is usually present along epineural surfaces and the adventitia of normal arteries and veins.
5.
An experienced assistant is an invaluable asset. For the academic surgeon, a senior resident or fellow is preferred; for the private practitioner, these lesions are usually not handled alone unless they are simple. Nothing eases a tedious dissection better than a pair of experienced hands to provide retraction, counter-traction, and control of unwanted bleeding. Strength and endurance of the assistant becomes important in the treatment of large, diffuse lesions of the chest wall, axilla, elbow, and palmar regions where the maintenance of surgical planes is essential.
6.
Identify and tag normal structures first, before diving into excision. Despite considerable distortion by the malformation, the normal limb anatomy remains constant. Nerves are recognized by the omnipresent perineural fat present even in malformed and densely scarred regions. This identification may be confusing and tedious in LMs, VMs, and mixed lesions because of the presence of abundant amounts of dysplastic adipose tissue. Colored vessel loops are used to encircle veins (blue), arteries (red), nerves (yellow), and tendons (white) for retraction to facilitate resection and easier identification in subsequent tourniquet runs of a long procedure.
7.
Move swiftly. The first 90 min of the first tourniquet run is the best time to complete the most difficult portion of the procedure, because the tissues have the least blood staining and swelling. The surgeon must move rapidly and confidently through the dissection. These lesions can be complicated and unforgiving; surgeons who stop frequently to teach, probe, ponder, and procrastinate will soon find themselves operating within a heavily bloodstained field. Sterling Bunnell aptly likened this experience to “operating in the bottom of an inkwell.”
8.
Thorough dissection limited to a specified area should be performed so that future reentry into a densely scarred bed will be unnecessary. In certain regions, limited staged excisions are better than one extensive and protracted dissection at the expense of increased blood loss or leaving behind abnormal tissue. For example, the digits should be debulked in stages whereas a single procedure is usually best for the dorsum of the hand and wrist and the palm. Additionally, the axilla, brachial plexus, and anterior surfaces of the elbow and wrist are preferably dissected in one operation when the neurovascular structures are undamaged and free of surgical scar. The palm of the hand can be approached more than once as long as a thorough dissection within each given region is performed and the tissues to be subsequently removed remain untouched.
9.
Avoid vascular compromise. Ironically, vascular insufficiency can exist in the presence of a vascular anomaly, especially fast-flow types. The dorsum of the hand has an axial blood supply above the overlying fascia, whereas the palm of the hand receives intermittent perforators from palmar arterial branches. Thus, flaps can be developed safely in the dorsum, but similar flaps are prone to ischemia in the palm. To avoid digital vascular compromise, only one-half of a digit should be dissected at a time. One or two large dorsal veins per finger should be preserved if possible.
10.
Avoid intraneural dissection whenever possible, despite gross involvement. Rare indications for a microscopic internal neurolysis are painful calcifications and/or symptomatic thrombosis. Although many vascular malformations and in particular the venous type are entangled in nerves, dissection often leaves in-continuity neuromas with partial or complete loss of distal sensory or motor function. The iatrogenic symptoms are usually much worse than the presenting ones. Neural wrapping with autogenous veins, acellular biologics, or other alloplastic materials is a helpful adjunct to any neuroplasty.
11.
Avoid partial dissections within large muscle groups. Removal of the entire muscle en bloc is preferred to avoid a secondary contracture of the entire musculotendinous unit. If more than half of a skeletal muscle is excised piecemeal, a secondary contracture is likely. VMs, mixed CLVMs, CAVMs, and LVMs are the most problematic lesions, whereas pure LMs may extend beneath the muscular fascia but expand along fascial planes and usually do not penetrate the muscles themselves.
12.
Soft tissue replacement. The major intraoperative dilemma is what to do when there is diffuse involvement of all tissue planes. The most satisfactory outcomes generally follow complete excision of the anomalous soft tissue within a given region with preservation of nerves, skeletal structures, and joints. Involved and excised muscle tendon units, skin, and subcutaneous tissue may be reconstructed and/or replaced. Open wounds and marginal skin require appropriate resurfacing. If skin grafting is not possible, the wound must be closed with local tissue or a free flap. Microvascular flap replacement provides the most durable and predictable outcome.
13.
Revascularization. At least one functional artery must supply the thumb or each digit. One of the major axial arteries to the wrist and hand should be preserved. Injury to critical vessels may be inadvertent or even planned in major resections. In fast-flow lesions with steal symptoms, even a successful procedure may not improve the distal circulation. Reconstruction with an autologous vein graft is a simple and straightforward solution for the skilled microsurgeon. It is important to place the proximal anastomosis outside the apparent zone of anomalous arterial channels.
14.
Meticulous and thoughtful closure is mandatory. In complex cases, a poorly planned or executed closure can be a major source of complications. It takes experienced eyes to identify poorly perfused skin and experienced hands to feel undue tension on flaps. Subcuticular closures with absorbable sutures are preferred in the arm, forearm, and dorsum of the hand. Careful eversion of glabrous skin wounds is performed with absorbable chromic sutures in children and nonabsorbable sutures in adults. Despite meticulous technique, any incision of skin containing LM may become hypertrophic.
15.
Drains should be used liberally, and delayed primary closure of the wound should be performed once considered. The liberal use of tissue sealant products is encouraged. Persistent postoperative bleeding is usually best treated with direct pressure, elevation, and immobilization, rather than by reexploration. Baseline coagulation studies should be performed for large/diffuse VM and LVM.
16.
Immobilization of the operated extremity. Postoperative management can be as important as preoperative planning and intraoperative execution. In the treatment of children with vascular anomalies, the postoperative lack or loss of immobilization is the single most frequent cause of wound dehiscence, maceration, and chronic infection. One of the many favorable aspects of operating on children, however, is their resistance to stiffness and joint contractures. Implementing postoperative rehabilitation is therefore not as important in children as it is in the adult patient.
17.
Be prepared to amputate. The surgeon who has the courage to treat complex lesions should also be prepared to amputate a nonfunctional or painful digit or limb after unsuccessful attempts at debulking and palliation. The most difficult decisions are in young children who do not verbalize their pain and some teenagers who have poor tolerance to pain and have not adapted well to their limb malformation. Amputation of painful or parasitic parts is often unnecessarily delayed either by the reluctant surgeons or anxious parents with unrealistic expectations.
18.
Follow–up evaluation should be performed compulsively at yearly intervals, regardless of the particular type of malformation. Large AVMs, LMs, or mixed lesions may be well tolerated by young children, only to become burdensome to teenagers because of their expansion, bulk, ulceration, and appearance or vascular steal symptoms. Early childhood, adolescence, pregnancy, and the use of high-estrogen antiovulant medication are times when change may occur in vascular malformations due to hormonal influences.
Surgical Resection by Anatomic Region of the Upper Extremity
Arm and Axilla
Vascular anomalies in the upper arm and axillary areas are usually in continuity with anomalies extending into the supraclavicular fossa, neck, and not uncommonly the mediastinum. The inability to use a pneumatic tourniquet makes resection in this region hazardous – intraoperative blood loss can be life-threatening. Interventional radiologic and endovascular laser approaches are therefore preferred.
If resection is required, careful preoperative planning, coordination with the anesthesiologist, and careful dissection with cell savers, blood products, and hypotensive anesthesia available should ensure a successful outcome. Quilting sutures and tissue sealants are helpful during closure, and drains may need to remain in place for weeks or months postoperatively. The child in Fig. 2 required all these considerations for his complicated upper limb and mediastinal LM. It is impossible to remove all pathologic tissue in this region; however, initial resection should be thorough because reentry into these areas is always accompanied by major bleeding, scarring, and subsequent contracture.
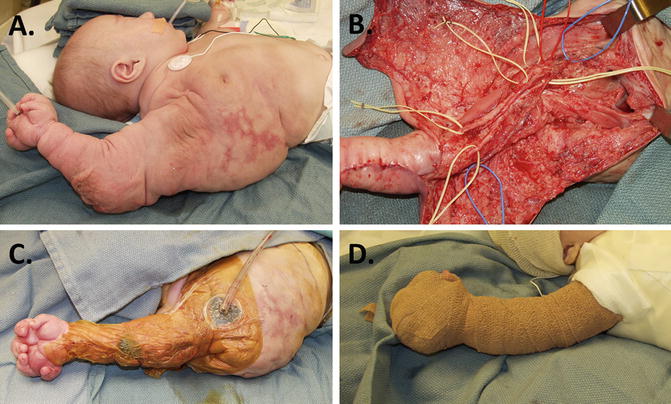

Fig. 2
Lymphatic malformation in upper limb/mediastinum. (a) Extensive LM diagnosed by prenatal ultrasonography; (b) aggressive debulking was performed at 6 months to control fluid shifts and massive size. Microvascular dissection preserved all neurovascular structures. (c) A vacuum-assisted (VAC) dressing was effective postoperatively; (d) correction maintained with Coban™ compression wraps
Large lymphatic channels or extensive VMs are often intertwined with the brachial plexus. The brachial plexus is easily damaged if one is not familiar with the anatomy, has inadequate hemostasis, or suboptimal assistance. Fortunately, an areolar/fat connective tissue plane is present around these nerves and serves as a guide for dissection.
All of these patients with large proximal lesions are at risk for pulmonary embolism even in the context of a normal preoperative coagulation work-up, since the vascular channels within the lesion – especially inflamed portions – may be locally coagulopathic.
Arm and Elbow
The lower arm and elbow region is much more amenable to surgical approaches than the axilla and upper arm because a pneumatic tourniquet can be used. Excision of an LM in this area is much easier than other types of malformation because LM is primarily confined to the subcutaneous tissue planes.
The neurovascular structures must be identified before the resection begins. They are generally in their normal location though they may course directly through extensive areas of malformation. The biceps tendon is the best reference point anteriorly, as the median nerve and brachial artery are always medial to it. Both these structures can be difficult to identify in the presence of an extensive vascular malformation, especially in combined lesions such as CLVM and LVM. It is very easy to inadvertently remove the brachial artery in the midst of large, thick-walled VM. Epineural fat is usually a different color from the dysplastic adipose tissue in the vascular malformation and is therefore a useful marker. The ulnar nerve should be transposed following debulking and decompression.
Proximal Forearm
Sclerotherapy is preferred in this location and remains the first line of intervention (Burrows and Mason 2004). If surgery is required, the forearm is ideally approached through a straight medial incision, extending from the wrist to the elbow. The proximal forearm presents one of the most difficult operative fields for the extremity surgeon due to the high density of important anatomic structures in a compact space. While LMs tend to stay between fascial planes, VMs in this region are usually diffuse and usually extend well beyond the fascial planes into the muscles. Intramuscular lesions are difficult to resect because of the potential for damage to adjacent muscle and injury to the many small motor branches of the median nerve. Commonly, vascular anomalies involve the dorsal interosseous system along the entire length of the interosseous membrane and may involve both the dorsal and the volar compartments.
Distal Forearm
The distal forearm and wrist regions are more amenable to surgical extirpation because there is less muscle and the major nerves have few arborizations. Surgical outcomes in this area are more predictable with less morbidity than outcomes following sclerotherapy (Upton et al. 1999; Upton and Marler 2006; Upton and Taghinia 2011). Frequently, what appears to be a superficial lesion in the distal forearm and wrist extend through the interosseous membrane and involve the rete system. Removal of the membrane may be required, but it is important to retain as much of the normal interosseous arterial anatomy as possible. The median and ulnar nerves are frequently involved in this region and may become heavily studded with nests of VM with intralesional thrombosis. Denervation of both anterior and posterior interosseous nerves may help alleviate postoperative wrist pain.
Hand
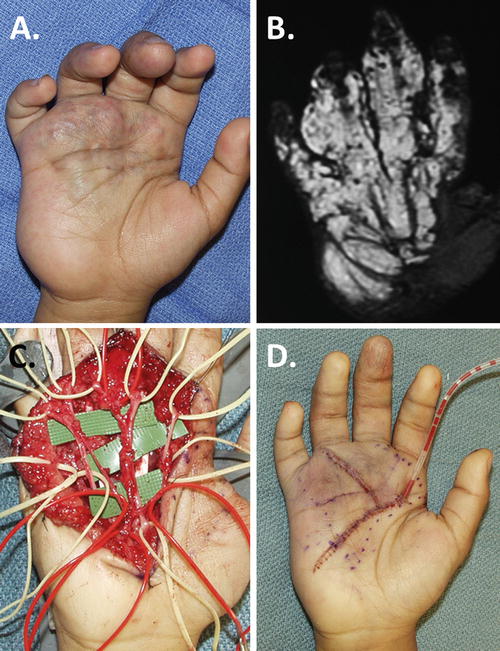
Sclerotherapy in this portion of the hand is highly discouraged because of the many potential complications due to the high concentration of neurovascular structures, gliding tendons, and delicate intrinsic muscles. Because of its complex anatomy, the hand is the most difficult region to resect any type of vascular malformation (Upton and Marler 2006). Table 5 outlines key principles to follow in the approach to surgical resection of palmar lesions.
Table 5
Caveats of palmar dissection
Anatomy complex. Have three-dimensional perspective |
Compulsive hemostasis under tourniquet. Avoid inkwell phenomenon |
Proximal to distal dissection of nerves. Look for epineural fat and follow epineural connective tissue plane |
Identify deep motor branch of ulnar nerve in Guyon’s canal and mid palm |
Median and ulnar nerves often involved. Take your time |
Although displaced, arterial is anatomy normal. Follow adventitial connective tissue plane. Save palmar arches |
Completely resect involved intrinsics to avoid contracture |
Try to preserve 1st dorsal interosseous and adductor pollicis muscles |
Simple muscle splitting for localized calcified thrombi |
Tissue sealants and drains to avoid bleeding |
Postoperative compression of thumb and digits easy with Coban |
Postoperative compression of palm difficult. Wrapping ineffective. Uniform pressure with VAC or dorsal/palmar splints |
Stay within a well-defined region. Tendency to be too aggressive |
Resect and replace marginal skin |
These procedures are tedious and lengthy, often requiring two or even three tourniquet runs. What appears to be localized lesions in the palm and dorsum of the hand is always found to be much more extensive intraoperatively. Technique must be meticulous, preserving all sensory and motor nerves, common digital arteries, and the palmar arch. Thorough exsanguination and compulsive hemostasis are mandated. Once blood staining occurs, the connective tissue planes become blurred. In Fig. 3, a typical palmar VM is presented with its preoperative bulk and blemish, an MR investigation of its extent, a precise dissection, and its immediate postoperative appearance and management with closed-suction drains.