and Nelson Claure2
(2)
Division of Neonatology, Department of Pediatrics, University of Miami Miller School of Medicine, Miami, FL, USA
48.1.1 Clinical Presentation
The original publication on bronchopulmonary dysplasia (BPD) by Northway and collaborators described a group of preterm infants who after prolonged mechanical ventilation developed chronic respiratory failure and characteristic radiographic findings (Northway et al. 1967). The lung damage was attributed primarily to the use of aggressive positive-pressure ventilation and high inspired oxygen concentrations. Today, with the widespread use of antenatal corticosteroids and the use of postnatal surfactant and less aggressive mechanical ventilation, this severe form of BPD has been replaced by a milder form that presents in the more immature infants who frequently have only mild initial respiratory disease (Charafeddine et al. 1999; Parker et al. 1992; Rojas et al. 1995). Therefore, these infants are not exposed to the very high airway pressures or oxygen concentrations, the two main factors in the pathogenesis of the original form of BPD. This milder form of the disease has been described as “New BPD.” This new presentation has created some inconsistencies and confusion in the definition and the diagnostic criteria of BPD (Bancalari et al. 2003).
The severe form of BPD was usually seen in infants who had severe respiratory failure from the time of birth and received aggressive ventilation and prolonged exposure to high inspired oxygen concentrations. These infants had characteristic changes in their chest radiographs and remained on ventilation and supplemental oxygen for long periods of time. This BPD was characterized by severe pulmonary damage that included emphysema, atelectasis, and fibrosis and marked epithelial squamous metaplasia and smooth muscle hypertrophy in the airways and in the pulmonary vasculature. These changes were associated with severe respiratory failure with airway obstruction, pulmonary hypertension, and cor pulmonale. This presentation is uncommon today, but there are still some infants who have this course and end up with severe respiratory failure, pulmonary hypertension, and marked alterations in their chest radiographs. The radiographs of infants with the milder forms of BPD show a more diffuse pattern reflecting loss of volume or increased lung fluid. Occasionally they also have dense areas of segmental or lobar atelectasis or pneumonic infiltrates but they do not show the areas of severe overinflation characteristic of the original BPD. These different clinical and radiographic manifestations reflect the different pathogenic process that underlines the new presentation of BPD.
This milder form of BPD seen more frequently today is characterized mainly by increased lung fluid, a diffuse inflammatory response, and by a striking decrease in alveolar septation and impaired vascular development (Abman 2000; Coalson et al. 1995; Husain et al. 1998; Jobe 1999; Margraf et al. 1991; Thibeault et al. 2003). These changes are more compatible with an arrest in lung development than with mechanical injury. It is not clear to what extent this arrest in lung development is secondary to the exposure of the premature lung to gas breathing versus the effects of overdistension and oxygen toxicity. Additional factors including incomplete lung development, inflammatory processes due to ante or postnatal infections, (Groneck et al. 1994; Groneck and Speer 1995; Hannaford et al. 1999; Pierce and Bancalari 1995; Watterberg et al. 1996; Yoon et al. 1997) and the exposure of the immature pulmonary vasculature to increased blood flow because of a persistent ductus arteriosus (Gonzalez et al. 1996; Marshall et al. 1999) are also implicated in the pathogenesis of the new BPD.
48.1.2 Definition
There has been a striking lack of uniformity in the diagnostic criteria for BPD among clinicians and in the literature. This explains some of the variation in the reported incidence of BPD among different centers. A major problem with most of the definitions of BPD is that they are based primarily on the need for supplemental oxygen that is used as a surrogate of the severity of the pulmonary damage. Supplemental oxygen is an important part in the management of these infants but in addition, it is implicated in the pathogenesis of BPD. Because there is no clear evidence on what the optimal arterial oxygen levels for these infants are, the indications for supplemental oxygen vary significantly from center to center. In addition, the need for supplemental oxygen can be influenced by altitude, drugs such as steroids or respiratory stimulants, and by the use of other forms of respiratory support such as nasal CPAP or mechanical ventilation. The proposed criteria to define BPD suggested in a National Institutes of Health (NIH) sponsored workshop in 1979 included a continued oxygen dependency during the first 28 days plus compatible clinical and radiographic changes (Bancalari et al. 1979). While these criteria were appropriate for the classic presentation of BPD, most infants today have intervals during the first days after birth when they do not require supplemental oxygen. Therefore, this criterion is not appropriate for the “New BPD.” To address this issue, some authors and clinicians have simplified this criterion and diagnose BPD in infants who are oxygen dependent “at” day 28. Although this simplified approach may work in most cases, it is possible that some infants who do not have significant lung disease may require supplemental oxygen only around day 28 because of acute conditions. This results in the erroneous labeling of those infants as BPD when in reality they do not have chronic lung damage. It is also possible that a premature infant may be on room air on day 28 and subsequently develop chronic lung damage and prolonged oxygen dependency. In order to avoid these problems, it is essential to include other indicators such as persistent radiographic changes and a minimal duration of oxygen therapy to reflect the chronicity of pulmonary damage.
The variation in the incidence of BPD when the different diagnostic criteria are applied to the same population of premature infants is illustrated in Fig. 48.1. Approximately one half of the infants who require oxygen at day 28 or for more than 28 days have a need for oxygen that persists to 36 weeks postmenstrual age (36 weeks PMA). Only a small fraction of infants from this cohort have an uninterrupted need for oxygen during the first 4 weeks. This accounts for the very low BPD incidence when using this definition. Although small in number, most infants who continuously require oxygen during the first 28 days go on to become oxygen dependent at 36 weeks PMA, very much like in the classic form of BPD. These infants, however, account only for a minority of infants who develop BPD today. Presently, most infants born ≤30 weeks of gestation do not have continuous oxygen need during the first month of life but still many of them go on to develop BPD. This presentation accounts for most of the BPD cases today.
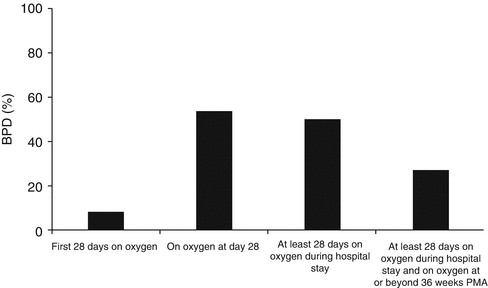

Fig. 48.1
Incidence of BPD using different definitions. Very few infants require oxygen continuously during the first 28 days after birth. In contrast, large proportions need oxygen at day 28 and for at least 28 days during their hospitalization. Only half of the latter group have a persistent need for oxygen at or beyond 36 weeks PMA (Data from 673 infants born at JMH, years 2004–2008; gestational age, 23–30 weeks; and alive at 36 weeks PMA)
In order to circumvent some of these problems, and to focus on the more severely afflicted infants, it has been proposed to use the need for supplemental oxygen at 36 weeks PMA as a better criterion for BPD (Shennan et al. 1988). Because this is a stricter criterion than the 28 days oxygen supplementation, it identifies a group of infants with more severe lung damage and therefore better predicts poor long-term outcome. The criterion of oxygen dependency at 36 weeks PMA requires for an infant born at 24 weeks of gestation to be on oxygen for 12 weeks, while an infant born at 32 weeks of gestation would be labeled as BPD if he or she requires oxygen for only 4 weeks after birth.
Figure 48.2 shows the incidence of BPD among infants born at Jackson Memorial Hospital (JMH) within different birth weight and gestational age strata according to the criteria of oxygen dependency for ≥28 days in combination with oxygen need at 36 weeks PMA.
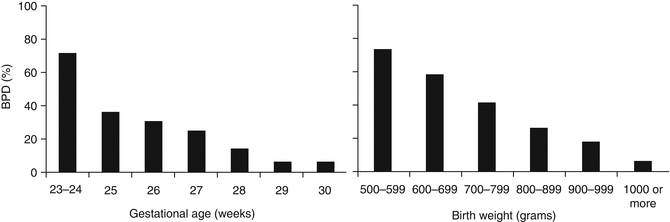

Fig. 48.2
Incidence of BPD by gestational age and birth weight. BPD defined as oxygen need for ≥28 days and at or beyond 36 weeks PMA (Data from 673 infants born at JMH during years 2004–2008; gestational age, 23–30 weeks; and alive at 36 weeks PMA)
In order to address the inconsistencies in the diagnostic criteria of BPD and to come up with a better definition a workshop was organized by the NIH in 2000 (Jobe and Bancalari 2001). As a result of this effort, the recommendation was to use oxygen need for ≥28 days and at 36 weeks PMA to identify different severity of BPD and also to use the oxygen concentration at 36 weeks PMA to further define the severity of lung injury. It was also agreed that a minimum of 28 days of supplemental oxygen was necessary to make the diagnosis of BPD (Table 48.1). These recommendations solve many of the problems with previous criteria but it still has the limitations of using 36 weeks PMA that were discussed earlier.
Table 48.1
NIH consensus definition of BPD
Gestational age | <32 weeks | ≥32 weeks |
|---|---|---|
Time point of assessment | 36 weeks PMA or discharge to home, whichever comes first | >28 days but <56 days postnatal age or discharge to home, whichever comes first |
Treatment with oxygen >21 % for at least 28 days plus | ||
Mild BPD | Breathing room air at 36 weeks PMA or discharge, whichever comes first | Breathing room air by 56 days postnatal age or discharge, whichever comes first |
Moderate BPD | Needa for <30 % at 36 weeks PMA or discharge, whichever comes first | Needa for <30 % at 56 days postnatal age or discharge, whichever comes first |
Severe BPD | Needa for ≥30 % oxygen and/or positive pressure (PPV or NCPAP) at 36 weeks PMA or discharge, whichever comes first | Needa for ≥30 % oxygen and/or positive pressure (PPV or NCPAP) at 56 days postnatal age or discharge, whichever comes first |
An important factor that is often ignored in the literature is the detailed and clear description of the base population in which the incidence of BPD is being reported. For example, reports that include only ventilated infants would have a higher rate of BPD than studies that include all live-born infants. Therefore, calculations of risk reduction and number needed to treat cannot be extended to the general population of infants even within the same range of birth weight or gestation. Benchmarking comparisons should also account for differences in the base population in regard to mortality. The reported incidence per total admissions may be much lower than the incidence among premature infant surviving the neonatal period, at 36 weeks PMA or at discharge. Inaccurate estimation of GA can also affect the calculated BPD incidence in a population where a consistent over- or underestimation of GA occurs.
Data combining results from competing outcomes are frequently found in the literature. For example, BPD or death, or survival without BPD is frequently reported as a combined outcome. This is done because BPD cannot be diagnosed in infants who die before the time point when diagnosis is made but it is essential that the outcomes are also reported separately. Regardless of the beneficial effect of a given intervention to prevent BPD, an increase in mortality or other serious complication beyond what is expected would nullify its use.
48.1.2.1 Prediction of Outcome
One of the main objectives of a diagnostic criterion for BPD is to predict the long-term pulmonary and neurodevelopmental outcome of preterm infants. The predictive value of the oxygen for ≥28 days, oxygen at 36 weeks PMA, and the NIH consensus definition was evaluated in a large cohort of infants from the National Institute of Child Health and Human Development (NICHD) neonatal network at 18–22 months corrected age (Ehrenkranz et al. 2005). The more liberal 28 days of oxygen criterion is more sensitive in detecting post-discharge respiratory complications but has poor specificity. Stricter definitions that use oxygen dependency at 36 weeks PMA and the more severe cases of need for ≥30 % oxygen are clearly more specific but at a cost of not classifying as BPD some infants that may later need additional respiratory care. These findings are similar to those reported from data obtained in the Trial of Indomethacin Prophylaxis in Preterms (TIPP) where oxygen need after day 28 was shown to be a more sensitive test (although less specific) than oxygen beyond 36 weeks PMA in predicting poor long-term pulmonary outcome (Davis et al. 2002). These results emphasize the importance of considering the respiratory evolution during the neonatal period in addition to the need for supplemental oxygen at a given point.
The BPD definition of oxygen at 36 weeks PMA is slightly more sensitive in predicting mental and psychomotor developmental impairment but the definition of severe BPD (oxygen ≥30 % at 36 weeks PMA) increases the predictive sensitivity. A recent multivariate analysis indicated an increased risk of poor neurologic outcome among infants deemed to have BPD based on their oxygen need at 36 weeks PMA (Schmidt et al. 2003). Interpretation of these findings however is difficult because of the confounding effects of some risk factors for BPD as they may also be independently associated with poor neurologic outcome.
48.1.2.2 Physiologic Definition
In order to reduce the influence of different strategies for oxygen supplementation on the reported incidence of BPD (Ellsbury et al. 2002), Walsh and collaborators have devised a physiologic test to standardize the need for oxygen at the time when BPD is being diagnosed (Walsh et al. 2004). To accomplish this, infants with moderate dependency on oxygen at 36 weeks PMA (<30 % oxygen) are challenged with room air breathing following a set weaning protocol to determine whether the supplemental oxygen is in fact needed. Although this test was applied in only 14 % of their entire cohort, it reduced the incidence of BPD from 35 to 25 % compared to the clinically prescribed oxygen supplementation. The use of this test clearly demonstrated that part of the reported variation in incidence of BPD among centers participating in the NICHD neonatal network was due to differences in clinical practice with respect to oxygen therapy. While the overall reduction in BPD incidence was 10 %, it ranged from 0 to 44 % in different centers demonstrating the wide variation in the use of supplemental oxygen. Over 40 % of the infants tested passed the challenge test and did not need the clinically prescribed oxygen.
Application of any diagnostic criteria also needs to consider other forms of respiratory support such as CPAP or IPPV. These interventions are included in the NIH consensus definition and can significantly influence the results of a diagnostic assessment and its relevance on outcome. However, they are rarely considered in the literature. Most definitions of BPD use supplemental oxygen as the main parameter to define chronic lung damage. This is associated with a number of limitations. Whether a small preterm infant remains on supplemental oxygen for 28 days or until 36 weeks PMA may not be very important because most of these infants remain hospitalized past the 36 weeks PMA for reasons other than their oxygen requirement. Therefore, the duration of oxygen therapy is not very important unless it predicts poor long-term outcome. Unfortunately, the reported predictive value of oxygen need in terms of long-term outcome has not been very strong.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


