Objective
The purpose of this study was to compare the chemotactic activity of the choriodecidua and amnion, and to identify the phenotype of the leukocytes chemoattracted by each tissue.
Study Design
Amnion, choriodecidua and whole fetal membranes extracts were obtained from women at term (>37 weeks of gestation) with or without labor (n = 5 each). Extracts were assayed for leukocyte chemotactic activity, and the number and phenotype of the chemoattracted leukocytes were characterized by flow cytometry.
Results
Although all of the extracts exhibited chemotactic activity, more leukocytes were chemoattracted by the choriodecidua and the whole fetal membranes during labor compared with no labor ( P = .010, .008). During labor the choriodecidua is responsible for granulocyte, T-lymphocyte, monocyte, and natural killer-cell chemoattraction, and the amnion is responsible for B-lymphocyte chemoattraction.
Conclusion
Choriodecidua and amnion exhibit chemotactic activity for selective leukocytes and thus, each fetal membrane differentially regulates leukocyte chemotactic activity during labor.
The process of normal labor in humans represents a type of inflammatory response. Many of the cellular and biochemical mediators of inflammation elicit specific responses in the quiescent uterus, the cervix, and the fetal membranes. As part of this process, leukocytes infiltrate the myometrium, the cervix, and the fetal membranes. These leukocytes, along with the resident cells of the reproductive tissues secrete inflammatory mediators, including cytokines, matrix metalloproteinases (MMPs), and prostaglandins (PGs), which participate in the regulation of the events of labor. Thus, it has been proposed that specific leukocyte subsets infiltrate the fetal membranes around the time of labor, creating an inflammatory microenvironment, and secreting mediators that promote the final rupture of these tissues. Granulocytes, T cells, and a lesser proportion of monocytes are generally considered to be the leukocyte species that infiltrate the fetal membranes. Each one of these leukocyte subpopulations has a specific role that we summarized in our recent published review.
Leukocyte recruitment and homing are regulated by specific mediators and generally occur in sequential steps. First, tissues secrete chemokines that are the soluble mediators that are responsible for the selective recruitment of leukocytes to a particular tissue. Once they have arrived at the tissue, leukocytes express cell adhesion molecules (CAMs), which allow them to adhere to the vascular endothelium and subsequently to extravasate from the blood vessel and into the tissue. In the reproductive tissues, it has been demonstrated that chemokines recruit specific leukocyte subsets before and during labor.
Direct evidence that chemokines actively participate in the recruitment of leukocytes to the fetal membranes (amnion and choriodecidua) during labor was recently provided. Products secreted by the fetal membranes induced selective recruitment of specific leukocyte subsets in vitro. This chemotactic activity was associated with the presence of specific chemokines, including chemokine C-X-C motif ligand (CXCL)8, CXCL10, chemokine C-C motif ligand (CCL)2 and CCL3, and cytokines, including tumor necrosis factor (TNF)-α and interleukin (IL)-1β. However, it is not known whether the amnion or the choriodecidua is responsible for this chemotactic activity, or if an interaction between the 2 membranes contributes to this process during labor. In this work, we hypothesize that the amnion and the choriodecidua secrete specific chemokines to recruit selective leukocyte subsets, and that both membranes work synergistically to mediate these processes during labor.
Materials and methods
This study was approved by the Institutional Review Board of the Instituto Nacional de Perinatologia Isidro Espinosa de los Reyes in Mexico City (Register 212250-02181). Written, informed consent was obtained from each subject before inclusion in the study.
Tissues
Fetal membranes were collected aseptically from 2 groups: (1) women with more than 37 weeks of gestation who had spontaneous active vaginal delivery (TL) developed (n = 5). Existence of labor was documented by cervical dilation (≥4 cm) and contractility of the myometrium (≥3 contractions of 40 seconds in 10 minutes by tocodynamometer) in the presence of spontaneous rupture of membranes (ROM). The duration of labor was similar between them (8-15 hours). (2) Women with more than 37 weeks of gestation who underwent elective cesarean section and did not develop active labor (TNL) (n = 5). Absence of cervical dilation, no uterine contractions, and integrity of the fetal membranes (absence of ROM) were documented. Women with premature ROMs and those with microbiologic or clinical evidence of cervicovaginal or intrauterine infection were excluded. Microbiologic tests were performed in tissues by rolling a Dacron swab on the surface of the membranes. The swabs were cultured onto blood agar plates under aerobic and anaerobic conditions to ensure that tissues were free from infection. Women included in this study had internal monitoring and they were similar in ethnicity (Mexican Mestizo) and parity (primiparous). None of these women received antibiotics for prolonged ROM, oxytocin augmentation, or immunosuppressive or modulating medications.
Fetal membrane extracts
Immediately after delivery, the fetal membranes from both groups of women were sterile dissected from the placental edge and placed in a sterile phosphate-buffered saline solution (1 times PBS). Within 30 minutes, membranes were washed thoroughly in sterile 1 times PBS to remove blood and debris. From each fetal membrane, we obtained random pieces for explants from the amnion, choriodecidua, and whole fetal membranes. We obtained 8-10 pieces each of 6.5 cm-diameter using a sterile circle borer and placed them in 75 cm 2 culture flasks. Immediately each explant was cultured (1 explant/1 flask) in 6.5 mL of Dulbecco-modified Eagle medium (DMEM), 1% MEM sodium pyruvate, and 1% antibiotic-antimycotic (100 U penicillin, 100 μg streptomycin, 0.25 μg amphotericin body mass index [BMl]) for 24 hours at 37°C in a humid atmosphere containing 5% CO 2 . Fetal calf serum-free conditions were used in all experiments. After this incubation, all explants were homogenized in their culture media using a Polytron (Brinkmann, Westbury, NY) and pooled. Fetal membrane extracts were centrifuged at 14,000× g , the supernatant was filtered through a 0.2 μm membrane (Corning, Corning, NY), and preserved at −70°C until use.
Leukocytes
Peripheral blood samples treated with heparin were collected from women fitting the same clinical characteristics as the groups described previously (TL or TNL, n = 5 each). Polymorphonuclear and mononuclear leukocytes were isolated using a Ficoll gradient (Polymorphoprep; Axis-Shield, Norton, MA). Total leukocytes were then washed and cultured in RPMI medium supplemented with 10% fetal calf serum (FCS) and 1% antibiotic-antimicotic and cultured for 24 hours at 37°C in humidified air containing 5% CO 2 . After incubation, leukocytes were extensively washed to eliminate FCS and suspended in the same FCS-free medium as described previously for the fetal membrane extracts. The number and viability of leukocytes were assessed by the trypan blue exclusion assay (all >95%). All culture reagents were purchased from GIBCO Invitrogen Corporation (Carlsbad, CA).
Chemotaxis assay
The chemotaxis assay was performed using a modified Boyden chamber (BY312; Neuro Probe, Gaithersburg, MD). Five hundred microliters of heterologous leukocyte suspension containing 500,000 leukocytes (1000 cells/mm 2 of filter area) were placed on top of the polycarbonate membrane (5 μm pore size; TMTP01300; Millipore Corporation, Billerica, MA) with either fetal membrane extract (amnion, choriodecidua, or whole fetal membranes) or medium control as the chemoattractant in the lower compartment. Leukocytes were matched in the chemotaxis assays, thus extracts from TNL or TL women were tested with leukocytes from TNL or TL women, respectively. Chambers were incubated for 90 minutes at 37°C in humidified air containing 5% CO 2 . Afterward, chemoattracted cells were removed from the lower compartment and centrifuged at 500× g for 5 minutes at room temperature. The pellet was stained and analyzed by flow cytometry to identify the phenotype of chemoattracted heterologous leukocytes using conjugated monoclonal antibodies: total leukocytes/CD45-FITC (PN IM2643), T-lymphocytes/CD3-PC7 (6607100), B-lymphocytes/CD19- PC5 (PN IM2643), monocytes/CD14-ECD (PN IM2707U), and natural killer (NK) cells/CD56-PE (PN IM2073) (Beckman Coulter, Brea, CA). We considered granulocytes as CD45 + CD3 − CD19 − CD14 − CD56 − cells. The flow cytometer (FC 500; Beckman Coulter) was set to analyze the samples for 300 seconds, which involved 10,000-100,000 events. The coefficient of variance of this method (inter- and intraassay) is <10%. We quantified the concentration of protein in the fetal membrane extracts using the Bradford Method and, the number of leukocytes that migrated was normalized by milligram of protein.
Statistical analysis
The data were examined initially by the Shapiro-Wilk test for normal distribution and were found to not be normally distributed. Therefore, the nonparametric Mann-Whitney test was used to differentiate statistical differences between groups (SPSS, version 16.0; SPSS, Inc, Chicago, IL). Significance was achieved with P ≤ .05.
Results
All fetal membrane extracts induced leukocyte chemotaxis. Whole fetal membrane extracts and separated choriodecidua extracts obtained from the TL group chemoattracted more leukocytes than those from the TNL group (n = 5; P = .01 and P = .008, respectively). Extracts from the amnion did not show differences between groups. Leukocyte chemotactic activity was greater in the choriodecidua extracts than in the amnion extracts obtained from TL tissues (n = 5; P = .016); however, there was no difference between these 2 groups in the TNL extracts. In addition, whole fetal membranes had higher chemotactic activity than the amnion in TL tissues (n = 5; P = .029). Our data also indicate that the amnion and choriodecidua extracts obtained from TNL tissues chemoattracted the same number of leukocytes, and their activity did not demonstrate an additive effect when the fetal membranes were together ( Figure 1 ).

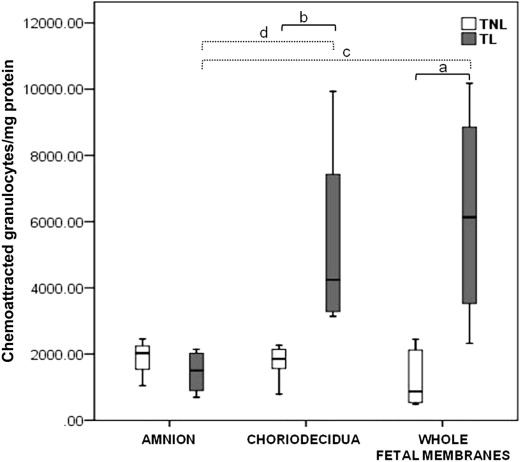
Granulocytes were the main subset chemoattracted by all extracts. Granulocyte chemoattraction was higher in TL extracts than in TNL extracts both in the choriodecidua ( P = .008) and the whole fetal membrane extracts ( P = .019). In the amnion extracts, there was no significant difference in granulocyte chemoattraction between TL and TNL tissues. The choriodecidua ( P = .016) and the whole membrane ( P = .029) extracts from TL tissues chemoattracted more granulocytes than the amnion ( Figure 2 ).

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


