Childhood Vaccines
Evridiki V. Fera
Paola J. Maurtua-Neumann
David W. Scheifele
Pearay L. Ogra
Introduction
Effective control or total elimination of many childhood infections by vaccinations is one of the most remarkable success stories of modern mankind. Immunization is also among the most cost-effective preventive measures available. Currently, about 24 bacterial and viral pathogens are the targets of immunization with specific vaccines in the United States and other parts of the world. These vaccines include replicating or nonreplicating antigens delivered via systemic intramuscular, subcutaneous, or mucosal (intranasal, oral) routes as shown in Table 57.1. To benefit fully from immunization, susceptible subjects need to receive the recommended vaccines at the recommended ages. This helps ensure protection against the peak risks of the target diseases. Of the vaccines available currently, most have been employed for Universal Childhood Vaccination Programs in different parts of the world. The recommended immunization schedules with vaccines licensed for children and adolescents in the United States for the year 2009 (1) are shown in Tables 57.2 to 57.4. This chapter provides comprehensive information about each of the available vaccines. This information is designed to meet the needs of a first-time or occasional vaccine provider. However, other health care providers who immunize very frequently may obtain additional information from other sources, such as the published statements of the Advisory Committee on Immunization Practices (ACIP) of the US Public Health Service (available online at www.cdc.gov/vaccines/), the “Red Book” from the American Academy of Pediatrics, the frequently updated Bulletins of the World Health Organization, and the Morbidity and Mortality Weekly Report (MMWR) from the Centers for Disease Control and Prevention (CDC), Atlanta, GA.
Anthrax Vaccine
The infection is caused by Bacillus anthracis, a spore-forming bacterium. It is a disease mainly of livestock. However, human infection may be acquired after exposure to infected animal products. The infection can be acquired through broken skin (cutaneous anthrax), by eating contaminated food (gastrointestinal anthrax), or by inhalation of anthrax spores (inhalation anthrax). Of the cases reported in the United States, 95% are cutaneous. The fatality rate for cutaneous anthrax is 5% to 20% without antibiotic treatment and less than 1% with antibiotic treatment. For those exposed through the digestive tract, the case fatality is estimated to be 25% to 60%. Before the availability of antibiotics or a vaccine, inhalation anthrax was almost always fatal. However, more recent experience suggests that early diagnosis and treatment significantly improves the prognosis and outcome.
Vaccine Preparations
Anthrax Vaccine Adsorbed (BioThrax) is a sterile, milky-white suspension (when mixed) made from cell-free filtrates of microaerophilic cultures of an avirulent, nonencapsulated strain of B. anthracis. The production cultures are grown in a chemically defined protein-free medium consisting of a mixture of amino acids, vitamins, inorganic salts, and sugars. The final product, prepared from the sterile filtrate culture fluid, contains proteins, including the 83 kDa protective antigen protein, released during the growth period. The final product is a nonreplicating bacterial product and is formulated to contain 1.2 mg per mL of aluminum, added as aluminum hydroxide in 0.85% sodium chloride. It also contains 25 μg per mL of benzethonium chloride and 100 μg per mL of formaldehyde, added as preservatives (1).
The current vaccine was licensed by the Food and Drug Administration (FDA) on the basis of its ability to prevent anthrax after preexposure immunization. Since anthrax immunization is considered an investigational use of an approved vaccine, informed consent is required.
Vaccine Usage
To prevent anthrax infection before exposure, the standard schedule consists of six doses administered subcutaneously. Following the first dose, additional doses of vaccine are given at 2 and 4 weeks, followed by booster doses 6, 12, and 18 months later. Finally, an annual booster dose is recommended to maintain prolonged immunity.
In one proposed study to prevent anthrax infection among postal workers and Capitol Hill employees who
may have already been exposed, recipients of the vaccine received a three-dose course with an interval of 2 weeks.
may have already been exposed, recipients of the vaccine received a three-dose course with an interval of 2 weeks.
Table 57.1 List of Currently Available Vaccines Against Childhood Infections | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
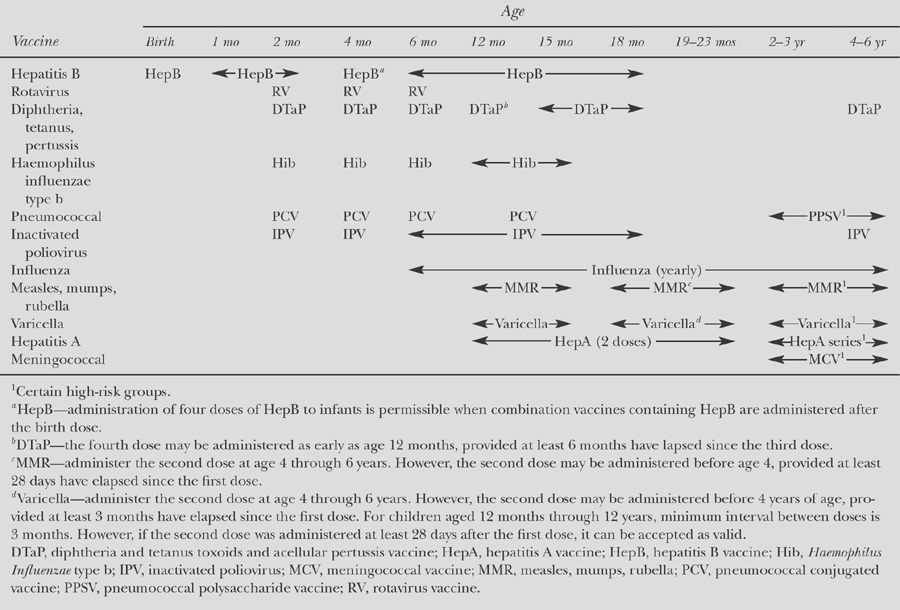
Table 57.2 Recommended Immunization Schedule for Persons Aged 0 Through 6 Years | |
|---|---|
|
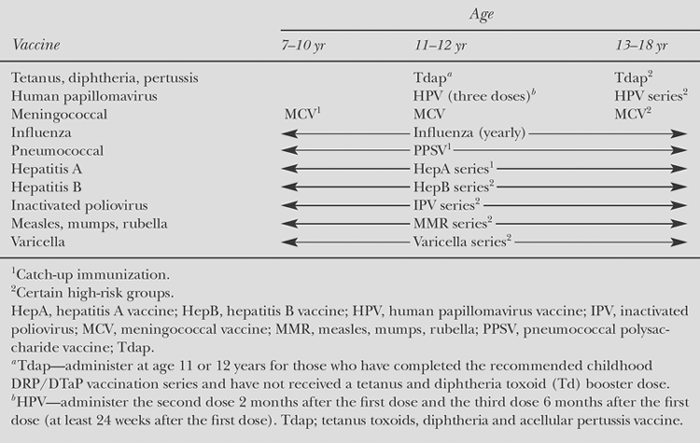
Table 57.3 Recommended Immunization Schedule for Persons Aged 7 Through 18 Years | |
|---|---|
|
Vaccine Effectiveness
Anthrax vaccine has been found to be 92.5% effective in protecting against anthrax (both cutaneous and inhalational).
As with most vaccines, measuring anthrax antibody levels provides only an indirect measure of the vaccine’s ability to prevent disease. However, 91% of adults who receive the anthrax vaccine exhibit an immune response after two or more doses, and 95% have a fourfold increase in antibodies after three doses. Higher levels of antibodies are thought to protect against anthrax, although the precise level of antibody at which protection against anthrax can be assured is not known.
The vaccine is the only aluminum-containing vaccine that is given subcutaneously. Aluminum is incorporated into many vaccines to increase their potency and produce the desired protection.
Anthrax vaccine has been recommended for use in the following groups of subjects at high risk for exposure: subjects 18 to 65 years of age who are at occupational risk of exposure to anthrax bacteria or spores; including at-risk veterinarians, livestock handlers, laboratory workers exposed to anthrax bacteria, and military personnel.
Adverse Effects and Contraindication
Anthrax vaccine may cause soreness, redness, itching, swelling, and lumps at the injection site. About 30% of men and 60% of women report mild local reactions, usually lasting for only a few days. Lumps can persist for a few weeks. Between 1% and 5% of those who receive the vaccine report moderate reactions (redness, swelling) of 1 to 5 inches in diameter. Larger reactions occur in 1% of vaccine recipients.
Some vaccinees experience rashes (16%), headaches (14% to 25%), joint aches (12% to 15%), malaise (6% to 17%), muscle aches (3% to 34%), nausea (3% to 9%), chills (2% to 6%), or fever (1% to 5%) after vaccination. These symptoms usually go away after a few days. Severe allergic reactions have been reported in less than 1 out of every 100,000 doses administered (1,2).
Concerns have been raised about potential long-term effects of anthrax vaccine and the overall safety of the vaccine. A March 2000 report by the Institute of Medicine’s Committee on Health Effects Associated with Exposures during the Gulf War noted that “to date, published studies have reported no significant adverse effects of the vaccine, but the literature is limited to a few short-term studies.”
A review of surveillance for adverse events following anthrax vaccination found that, although there are short-term side effects—more common among women than men—“no patterns of unexpected local or systemic adverse events have been identified.”
Anthrax vaccine is not recommended for postexposure protection, subjects who have recovered from an anthrax infection, and subjects who developed a serious allergic (anaphylactic) reaction to a previous dose of anthrax vaccine.
The vaccine is not indicated for preexposure protection in pregnant women, as the vaccine has not been proven to be safe in such situations, and for subjects younger than 18 years or older than 65 years, because no formal studies have been completed in these groups; subjects moderately or severely ill should consult with their physicians before receiving such vaccine.
Table 57.4 Catch-Up Immunization Schedule for Persons Aged 4 Months Through 18 Years | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Diphtheria Toxoid
The etiologic agent of diphtheria is Corynebacterium diphtheriae. The most common manifestation of diphtheria is severe pharyngitis. This is caused by a toxin released by the infecting organism that damages the respiratory epithelium, causing pseudomembrane formation. Toxin dissemination can also lead to nerve and cardiac muscle damage. Without treatment mortality is high particularly as a result of airway obstruction or cardiac dysfunction. Transmission occurs from person to person by close respiratory and physical contact.
Vaccine Preparations
The vaccine (3) is based on diphtheria exotoxin, a protein that is antigenically uniform among isolates. In the manufacturing process, organisms are grown under conditions favoring toxin production. Free toxin is separated from bacteria by centrifugation and then progressively purified. Controlled exposure to formalin alters the protein structure sufficiently to abrogate toxicity while preserving antigenicity, producing a so-called toxoid. Diphtheria toxoid is usually adsorbed to an aluminum salt to enhance immunogenicity over fluid formulations. Formulations intended for young children contain more antigen (typically 6.7 to 15 Lf units per 0.5 mL dose) than formulations intended for persons older than 6 years (typically 2 Lf units per 0.5 mL dose). To make this distinction, the abbreviation D toxoid is used for pediatric formulations and the abbreviation d toxoid is used for “adult” formulations. Some products contain thimerosal preservative.
No diphtheria-only vaccine is available. Currently, the toxoid-containing vaccine includes DTaP (Diphtheria–Tetanus–acellular Pertussis) vaccine, in combination with Haemophilus influenzae type b (Hib) vaccine, in combination with hepatitis B and inactivated polio vaccines, in combination with Hib, hepatitis B, and inactivated polio vaccines, DT or Td (in combination with tetanus vaccine), and Tdap (Diphtheria–Tetanus–Pertussis).
Vaccines containing the whole cell pertussis component (DTP) are no longer recommended for use in the United States and are not listed here, although they are used in many other countries. Vaccines containing lower amounts of diphtheria toxoid—abbreviated with a small d—are utilized in persons aged 7 years or older. Pertussis component–containing vaccines are not available for children 7 to 9 years of age.
Available Products
Product Name: Diphtheria and tetanus toxoids adsorbed (DT)
Manufacturer: Sanofi Pasteur
Year Licensed: 1984
Product Name: Tetanus and diphtheria toxoids adsorbed for adult use (Td)
Manufacturers (Year Licensed): Massachusetts Public Health Biologic Laboratories (1970), Aventis Pasteur (1978)
Product Name: Tripedia (DTaP)
Manufacturer: Sanofi Pasteur
Year Licensed: 2001
Product Name: Infanrix (DTaP)
Manufacturer: GlaxoSmithKline
Year Licensed: 1997
Product Name: TriHIBit (DTaP and Hib conjugate vaccine)
Manufacturer: Sanofi Pasteur
Year Licensed: 2001
Product Name: DAPTACEL (DTaP)
Manufacturer: Sanofi Pasteur
Year Licensed: 2002
Product Name: Pediatrix (DTaP, hepatitis B, and inactivated polio vaccines)
Manufacturer: GlaxoSmithKline
Year Licensed: 2002
Product Name: DECAVAC (Tetanus and Diphtheria Toxoids Adsorbed for Adult Use—preservative free)
Manufacturer: Sanofi Pasteur
Year Licensed: 2004
Product Name: BOOSTRIX (Tetanus Toxoid, Reduced Diphtheria Toxoid and Acellular Pertussis Vaccine, Adsorbed for use in 10- to 64-year-old persons) (Tdap)
Manufacturer: GlaxoSmithKline Biologicals
Year Licensed: 2005
Product Name: ADACEL (Tetanus and Diphtheria Toxoids Adsorbed for use in 11- to 64-year-old persons)
Manufacturer: Sanofi Pasteur
Year Licensed: 2005
Product Name: Pentacel (DTaP, Hib conjugate, hepatitis B, and inactivated polio vaccines)
Manufacturer: Sanofi Pasteur
Year Licensed: 2008
All DTaP vaccines are available containing no or only trace amounts of thimerosal. Both DT and Td are available as vaccines, containing trace amounts of thimerosal, or as thimerosal-free. Tetanus toxoid (TT) is only available containing thimerosal preservative.
Vaccine Usage
Adsorbed toxoid formulations are administered intramuscularly to minimize injection site reactions. Vaccine should be stored at 2#65518;C to 8#65518;C, avoiding freezing.
Diphtheria toxoid requires a series of appropriately spaced injections to reliably elicit a protective response. The number of doses needed to elicit protection varies with age. Antibody levels decline slowly and may fall below the minimum needed for protection. Periodic booster doses are recommended throughout life to maintain protection.
The recommended immunization schedule for infants and young children in the United States (3) and Canada (4) involves five doses of the pediatric (D) formulation, given at 2, 4, 6, and 15 to 18 months and 4 to 6 years. Subsequent booster doses are recommended at 14 to 16 years and every 10 years throughout adulthood, using the adult (d) formulation (see Tables 57.2 to 57.4).
Vaccine Effectiveness
Diphtheria vaccines have been routinely used since the 1930s. Clinical diphtheria is rare in well-immunized populations. Although the vaccine induces only antitoxic immune responses, prolonged use in populations has resulted in the near disappearance of circulating toxigenic organisms. In the United States, no more than five respiratory cases are reported annually, most of whom lacked adequate primary immunization. A substantial proportion of American and Canadian adults lack protective serum levels of antitoxin from not having obtained booster immunizations, but disease is rare nonetheless. Persons likely to encounter diphtheria during travel abroad or who are exposed to a case should receive a booster dose if more than 10 years has elapsed since the previous dose (and the individual had adequate primary immunization).
Adverse Effects and Contraindications
Diphtheria toxoid vaccines are well tolerated. Occasionally, the vaccine can cause mild to moderate injection-site discomfort and low-grade fever. Booster doses may cause greater injection-site reaction with redness, swelling, and soreness. Overly frequent doses should be avoided, as they increase the risk of severe injection-site reactions. The only contraindication to its use is a history of anaphylactic reaction to a previous dose or hypersensitivity to thimerosal (thimerosal-free formulations are available) or any other vaccine component.
Haemophilus Influenzae Type B Vaccines
Before vaccines were introduced in the late 1980s, Hib bacteria were the leading cause of purulent meningitis and epiglottis and an important cause of bacteremia, pneumonia, septic arthritis, and cellulitis in young children (5). About 1 child in 200 experienced invasive Hib infection by 5 years of age, half suffering from meningitis. The latter was the leading cause of acquired deafness and developmental delay. Hib organisms most often colonize the upper airway without causing disease and spread readily from child to child in respiratory secretions.
Immunity is associated with opsonic antibodies directed at the Hib capsular polysaccharide, polyribosyl ribose phosphate (PRP). Children do not respond to purified PRP until about 24 months of age, by which time about two-thirds of Hib cases have occurred. Response to PRP develops without T-cell help and lacks a memory component.
Vaccine Preparations
To protect the age group at greatest risk, Hib vaccines have to overcome the unresponsiveness to purified PRP characteristic of children younger than 24 months. This is achieved by chemically linking segments of PRP to the surface of carrier proteins, creating a polysaccharide–protein conjugate vaccine. Currently used carriers include tetanus toxoid (PRP-T), genetically inactivated diphtheria toxin (PRP-CRM), and outer membrane protein complex from Neisseria meningitidis (PRP-OMP). Uptake and processing of the linked molecules by antigen-presenting cells expose B cells responding to PRP to the cytokines released by T cells
responding to carrier peptides. The T-cell help extended to B cells responding to PRP fundamentally alters the response to the polysaccharide, enabling it to develop in young infants with additional advantages of antibody avidity maturation and development of immunologic memory. The latter establishes long-term protection and the capacity for booster responses to native PRP, should individuals be colonized with Hib or cross-reacting organisms.
responding to carrier peptides. The T-cell help extended to B cells responding to PRP fundamentally alters the response to the polysaccharide, enabling it to develop in young infants with additional advantages of antibody avidity maturation and development of immunologic memory. The latter establishes long-term protection and the capacity for booster responses to native PRP, should individuals be colonized with Hib or cross-reacting organisms.
Current vaccines contain purified PRP conjugated to carrier proteins that vary among manufacturers. Manufacturing processes control the size and quantity of PRP molecules linked to the carrier protein, minimizing lot-to-lot variation in immunogenicity. Products are available in separate formulations or combined with other childhood vaccines.
The Hib vaccine is available as Hib (alone), Hib in combination with DTaP vaccine, and Hib in combination with recombinant hepatitis B virus (HBV) vaccine.
Available Products
Product: ActHIB (Hib)
Manufacturer: Aventis Pasteur
Year Licensed: 1993
Product: HibTITER (Hib)
Manufacturer: Wyeth Lederle
Year Licensed: 1990
Product: PedvaxHIB (Hib)
Manufacturer: Merck
Year Licensed: 1989
Product: Comvax (HBV-Hib)
Manufacturer: Merck
Year Licensed: 1996
Vaccine Usage
A series of injections in the first months of life is required to elicit an antibody response. Products requiring three primary doses are given at 2, 4, and 6 months of age (see Table 57.2). Of note, PedavaxHIB by Merck requires only two primary doses, given at 2 and 4 months of age. For each product a booster dose is recommended in the second year of life. The PRP–OMP vaccine elicits the quickest response and is preferred in settings where the disease risk is high in early infancy, as in Alaskan natives (2). Fewer doses are required for children who commence immunization after 6 months of age. Booster doses later in childhood (beyond 2 years) are not required (see Tables 57.2 to 57.4).
Immunization elicits anti-PRP antibodies. The minimum protective antibody level using conjugate vaccines has not been defined, although a level of 0.15 μg per mL or more is indicative of adequate protection. The capacity for an anamnestic response upon encountering organisms may be more relevant to protection than a particular serum antibody concentration.
Vaccine Effectiveness
Virtually every healthy infant has a measurable antibody response upon completion of the recommended primary series. Antibody concentrations decline progressively thereafter but are strongly reinforced by the booster dose. With the currently used vaccines, annual Hib case totals are less than 1% of what they were before immunization became routine (6). Among the remaining cases, vaccine failures are rare; cases more often reflect incomplete immunization and parent refusal to immunize. All current products have an estimated efficacy of at least 98%.
Adverse Reactions and Contraindications
Hib conjugates cause only minor adverse effects, such as fever and injection-site morbidity, of short duration. Local reactions do not increase with successive doses. Allergy to the carrier protein and an anaphylactic reaction to a previous dose are contraindications.
Hepatitis A Vaccine
Hepatitis A virus (HAV) causes most cases of acute hepatitis in the United States (7). Young children are often mildly ill or asymptomatic but can readily spread infection to contacts. In older children, teens, and adults, HAV infection causes substantial morbidity for 1 to 3 months (2). Infection superimposed on chronic liver disease increases the risk of fulminant hepatitis or liver failure. No chronic infection state occurs. Infection spreads through the fecal route, through direct contact with cases, or through ingestion of contaminated food or water. Recovery is associated with lifelong protection.
Vaccination programs target individuals at high risk of infection and communities with high rates or recurrent epidemics of infection (8).
Vaccine Preparations
Licensed vaccines contain inactivated HAV (9). The various vaccine strains are grown in human fibroblast cultures, purified from lysed cells, inactivated with formalin, and adsorbed to alum adjuvant. Pediatric formulations contain less antigen than do adult formulations and are intended for children older than 12 months (9). Some products may contain neomycin and other excipients.
The hepatitis A vaccine is available as HAV alone and HAV in combination with hepatitis B virus (HBV) vaccine (see Table 57.6). HAV vaccines are administered intramuscularly. They should be stored at 2#65518;C to 8#65518;C, avoiding freezing.
Available Products
Product: Havrix (HAV)
Manufacturer: GlaxoSmithKline
Year Licensed: 1995
Product: Vaqta (HAV)
Manufacturer: Merck
Year Licensed: 1996
Product: TwinRix (combined HAV, HBV)
Manufacturer: GlaxoSmithKline
Year Licensed: 2007
Table 57.5 Combination Vaccines | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Vaccine Usage
Hepatitis A vaccine was introduced incrementally first in 1996 for children living in communities with the highest rates of disease and then in 1999 for children living in states/communities with consistently elevated rates of infection. The impact of immunization with hepatitis A vaccine has been a dramatic decline in the rates of disease and a sharp reduction in the groups with the highest risk of infection, Native Americans and Alaskan natives. Rates of hepatitis A infection are now similar in most areas of the United States. As a consequence, hepatitis A vaccine has now been recommended for all children in the United States 12 to 23 months of age to eliminate hepatitis A transmission nationally. A single dose elicits protective levels of antibody within 4 weeks in more than 95% of healthy recipients (10). A second dose is recommended 6 to 12 or 6 to 18 months later (depending on the product) for long-term protection. Immunity persists for at least 10 years and is expected to last much longer (11).
Universal HAV immunization of children is recommended in states or communities with higher-than-average rates of infection (10).
Individual candidates for HAV immunization include the following: children traveling to countries where HAV infection is endemic; youth at risk through use of illicit drugs in unsanitary conditions, or involvement in the sex trade or male homosexual activities, particularly involving oral–anal contact; children who have clotting factor disorders treated with clotting-factor concentrates; and children with chronic liver disease or a transplanted liver.
HAV vaccine can be given concurrently with other childhood vaccines at separate anatomic injection sites. HAV vaccine can be used to prevent infection in persons recently exposed (within 7 days) to a case and appears to be as effective as immunoglobulin.
Vaccine Effectiveness
HAV vaccines are highly effective. Universal childhood immunization programs have been shown to substantially reduce infection rates in communities across all age groups, illustrating that children are central in the transmission of this virus. Vaccination is also effective for controlling outbreaks of infection (10).
Adverse Effects
HAV vaccines are well tolerated, with symptoms usually limited to soreness or erythema at the injection site. Rare instances of anaphylaxis have been reported. HAV vaccine should not be given to children who have had an anaphylactic reaction to a previous dose or are allergic to any constituent (e.g., neomycin). Safety of HAV administration during pregnancy has not been demonstrated.
Hepatitis B Vaccine
The hepatitis B virus (HBV) is a DNA virus which is an important cause of liver disease that ranges in severity from a mild illness lasting a few weeks (acute) to a serious long-term illness that can lead to chronic active hepatitis or liver cancer. This virus can be transmitted through contact with infectious blood, semen, and other body fluids from having sex with an infected person, sharing contaminated needles to inject drugs, or from an infected mother to her newborn (2).
The virus contains several important antigenic components. These include hepatitis B surface antigen (HBsAg), hepatitis B core antigen (HBcAg), and hepatitis B e antigen (HBeAg). Humans are the only known host for HBV, although some nonhuman primates have been infected in laboratory conditions. HBV is relatively resilient and, in some instances, has been shown to remain infectious on environmental surfaces for more than 7 days at room temperature. HBV infection is an established cause of acute and chronic hepatitis and cirrhosis. It is the cause of up to 80% of hepatocellular carcinomas and is second only to tobacco among known human carcinogens (2).
Vaccine Preparation
Hepatitis B vaccine (Recombinant) is a noninfectious recombinant DNA hepatitis B vaccine (12). It contains purified HBsAg of the virus obtained by culturing genetically engineered Saccharomyces cerevisiae cells, which carry the surface antigen gene of the HBV. The surface antigen expressed in S. cerevisiae cells is purified by several physiochemical steps and formulated as a suspension of the antigen adsorbed on aluminum hydroxide. The preparations contain no more than 5% yeast protein. No substances of human origin are used in its manufacture.
Available Products
Single-antigen hepatitis B vaccines
Product: ENGERIX-B (HBV)
Manufacturer: GlaxoSmithKline
Year Licensed: 2006
Product: RECOMBIVAX-HB (HBV)
Manufacturer: Merck
Year Licensed: 2007
Combination Vaccines
Combined hepatitis B-Hib conjugate vaccine. Cannot be administered before the age of 6 weeks or after the age of 71 months (see Table 57.5).
PEDIATRIX: Combined hepatitis B, DTaP, and inactivated poliovirus (IPV) vaccine. Cannot be administered before the age of 6 weeks or after the age of 7 years (see Table 57.5).
TWINRIX: Combined hepatitis A and hepatitis B vaccine. Recommended for persons aged 18 years or older who are at increased risk for both HAV and HBV infections (see Table 57.6) (13).
Vaccine Usage
Each 0.5 mL dose contains 10 μg of HBsAg adsorbed on 0.25 mg aluminum as aluminum hydroxide. The pediatric formulation contains sodium chloride (9 mg per mL) and
phosphate buffers (disodium phosphate dehydrate, 0.98 mg per mL; sodium dihydrogen phosphate dehydrate, 0.71 mg per mL).
phosphate buffers (disodium phosphate dehydrate, 0.98 mg per mL; sodium dihydrogen phosphate dehydrate, 0.71 mg per mL).
Table 57.6 Combination Vaccines | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Vaccine Effectiveness
After three intramuscular doses of hepatitis B vaccine, more than 90% of healthy adults and more than 95% of infants, children, and adolescents (from birth to 19 years of age) develop adequate antibody responses. However, there is an age-specific decline in immunogenicity. After the age of 40 years, approximately 90% of recipients respond to a three-dose series, and by 60 years, only 75% of vaccines develop protective antibody titers.
All infants should receive the hepatitis B vaccine series as part of the recommended childhood immunization schedule. Primary vaccination consists of three or more intramuscular doses of hepatitis B vaccine. The second and third doses are administered 2 and 6 months, respectively, after the first dose (see Table 57.2). Alternate schedules have been approved for certain vaccines and/or populations (see Tables 57.2 to 57.4).
Adverse Reactions
Hypersensitivity to any component of the vaccine, including yeast, is a contraindication. This vaccine is contraindicated in patients with previous hypersensitivity to any hepatitis B–containing vaccine. As with other vaccines, vaccination of persons with moderate or severe acute illness, with or without fever, should be deferred until the acute phase of the illness is resolved. Vaccination is not contraindicated in persons with a history of multiple sclerosis, Guillain–Barré syndrome (GBS), autoimmune disease (e.g., systemic lupus erythematosus or rheumatoid arthritis), or other chronic diseases (11).
Pregnancy is not a contraindication to vaccination. Limited data indicate no apparent risk for adverse events
to developing fetuses when hepatitis B vaccine is administered to pregnant women. Current vaccines contain noninfectious HBsAg and should cause no risk to the fetus (11).
to developing fetuses when hepatitis B vaccine is administered to pregnant women. Current vaccines contain noninfectious HBsAg and should cause no risk to the fetus (11).
Management of Infants Born to Women Who Are HBsAg Positive or Unknown
All infants born to HBsAg-positive women should receive single-antigen hepatitis B vaccine and HBIG (0.5 mL) at 12 hours of birth or earlier, administered at different injection sites. The vaccine series should be completed according to a recommended schedule for infants born to HBsAg-positive mothers. The final dose in the vaccine series should not be administered before the age of 24 weeks (164 days).
For preterm infants weighing less than 2,000 g, the initial vaccine dose (birth dose) should not be counted as part of the vaccine series because of the potentially reduced immunogenicity of hepatitis B vaccine in these infants; three additional doses of vaccine (for a total of four doses) should be administered beginning when the infant reaches the age of 1 month (2).
Postvaccination testing for anti-HBs and HBsAg should be performed after completion of the vaccine series, at the age of 9 to 18 months (generally at the next well-child visit). Testing should not be performed before the age of 9 months to avoid detection of anti-HBs from HBIG administered during infancy and to maximize the likelihood of detecting late HBV infection. Anti-HBc testing of infants is not recommended because passively acquired maternal anti-HBc might be detected in infants born to HBV-infected mothers till the age of 24 months.
HBsAg-negative infants with anti-HBs levels of 10 mIU per mL or more are protected and need no further medical management.
HBsAg-negative infants with anti-HBs levels of less than 10 mIU per mL should be revaccinated with a second three-dose series and retested 1 to 2 months after the final dose of vaccine.
Infants who are HBsAg-positive should receive appropriate follow-up.
Infants of HBsAg-positive mothers may be breast-fed beginning immediately after birth.
Although not indicated in the manufacturer’s package labeling, HBsAg-containing combination vaccines may be used for infants aged 6 weeks or older born to HBsAg-positive mothers to complete the vaccine series after receipt of a birth dose of single-antigen hepatitis B vaccine and HBIG (2).
Women admitted for delivery without documentation of HBsAg test results should have blood drawn and tested as soon as possible after admission.
While test results are pending, all infants born to women without documentation of HBsAg test results should receive the first dose of single-antigen hepatitis B vaccine (without HBIG) at 12 hours of birth or earlier.
If the mother is determined to be HBsAg-positive, her infant should receive HBIG as soon as possible but no later than the age of 7 days, and the vaccine series should be completed according to the recommended schedule for infants born to HBsAg-positive mothers.
If the mother is determined to be HBsAg-negative, the vaccine series should be completed according to a recommended schedule for infants born to HBsAg-negative mothers.
If the mother has never been tested to determine her HBsAg status, the vaccine series should be completed according to a recommended schedule for infants born to HBsAg-positive mothers. Administration of HBIG is not necessary for these infants.
Because of the potentially decreased immunogenicity of vaccine in preterm infants weighing less than 2,000 g, these infants should receive both single-antigen hepatitis B vaccine and HBIG (0.5 mL) if the mother’s HBsAg status cannot be determined at 12 hours of birth or earlier. The birth dose of vaccine should not be counted as part of the three doses required to complete the vaccine series; three additional doses of vaccine (for a total of four doses) should be administered according to a recommended schedule on the basis of the mother’s HBsAg test result (2,11).
Human Papillomavirus
Human papillomaviruses (HPVs) are a group of more than 120 different virus types. Approximately 40 HPV types are primarily sexually transmitted from person to person (e.g., genital–genital contact, oral–genital contact, and sexual intercourse), infecting the oral, anal, or genital areas of both men and women. Genital HPV infections are very common: by 50 years of age, 70% to 80% of women and a similar percentage of men will have acquired genital HPV infection. Most genital HPV infections cause no symptoms and are cleared by the immune system within a few weeks or months.
Thus, the vast majority of people recover from genital HPV infection uneventfully. However, some people develop persistent or chronic genital HPV infection, which can lead to development of genital warts and anogenital cancers, especially cervical cancer.
Types 16 and 18 and others, known collectively as “high-risk” HPV types, may cause abnormal Pap tests and cervical cancer in women. Together types 16 and 18 cause approximately 70% of the cases of cervical cancer in the United States. Although there are a number of other risk factors for cervical cancer, being infected with a “high-risk” type HPV appears to be necessary to develop cervical cancer.
In both men and women, “high-risk” HPV infections are also thought to cause 85% of anal cancers, 50% of other anogenital cancers, 20% of cancers of the throat and mouth, and 10% of cancers of the larynx and esophagus.
Vaccine Preparation
Safe and effective virus-like-particle–derived recombinant vaccines have been developed against several HPV types associated with cervical cancer and genital warts. The HPV vaccine does not contain thimerosal.
Available Products
Product: Gardasil
Manufacturer: Merck
Year Licensed: 2006
A Quadrivalent Human Papillomavirus (Types 6, 11, 16, 18) Recombinant Vaccine was licensed by the FDA in 2006 for use in the United States.
Product: Cervarix (Types 16, 18)
Manufacturer: GlaxoSmithKline
Year Licensed in USA: 2009
Another vaccine is based on virus-like-particle of types 16 and 18 licensed by the FDA in 2009. Studies are currently in progress evaluating a vaccine consisting of large number of HPV types.
Vaccine Effectiveness
The efficacy of the HPV vaccine has been studied in women 16 to 26 years of age. In women who previously had not been exposed to the HPV types in the vaccine, the vaccine was 100% effective in preventing cervical precancers caused by the targeted HPV types and was nearly 100% effective in preventing vulvar and vaginal precancers and genital warts caused by the targeted HPV types.
If a female already has chronic infection with one of the HPV types, the vaccine will not prevent disease from that type.
Studies have shown that more than 99% of study participants developed antibodies after vaccination; antibody titers were higher for young girls than for older females participating in the efficacy trials. Routine vaccination against HPV is recommended for all girls 11 to 12 years of age. The vaccination series can be started in girls as young as 9 years of age. Catch-up vaccination is recommended for females 13 to 26 years of age who have not been vaccinated previously or who have not completed the full vaccine series, whether or not they have had sexual intercourse or previous evidence of HPV infection.
Women who are breast-feeding can receive the HPV vaccine. Moreover, immunocompromised women (from disease or medication) can receive the vaccine. However, the immune response to vaccination and vaccine effectiveness might be less than in women who are not immunocompromised.
Currently available HPV vaccine is not recommended for pregnant women since data on vaccination during pregnancy are limited. However, there is no evidence of risk to the fetus when a pregnant woman is inadvertently vaccinated; the manufacturer is maintaining a registry of pregnancy outcomes for this circumstance. Subjects with a history of immediate hypersensitivity to yeast or to any vaccine component should not receive the vaccine. Subjects with minor illnesses (e.g., diarrhea or mild upper respiratory tract infections, with or without fever) can receive the vaccine. However, those with moderate or severe acute illnesses should be deferred until after the illness improves.
Vaccine Usage
Each dose of quadrivalent HPV vaccine is 0.5 mL, administered intramuscularly. It should be administered in a three-dose schedule (see Table 57.3). The second and third doses should be administered 2 and 6 months after the first dose. The quadrivalent HPV vaccine can be administered at the same visit when other age appropriate vaccines are provided, such as Tdap, Td, and MCV4.
Adverse Effects
The HPV vaccine has been tested to date in more than 11,000 women (9 to 26 years of age) in many countries around the world, including the United States. These studies found that the HPV vaccine was safe and caused no serious side effects. Vaccine recipients experienced occasional pain, swelling, and redness at the injection sites.
Because these vaccines will only prevent infection with the two types of HPV that cause most cases of cervical cancer and the two types that most commonly cause genital warts, they are not expected to eliminate cancer nor genital warts related to other HPV types. Therefore, Pap screening and treatment programs for cervical cancer will still be needed. These vaccines are preventive and are expected to have no effect on preexisting infection with these HPV types. How long the vaccines will protect those who have been immunized remains to be determined (16,17).
Influenza Virus Vaccine
Influenza virus infections affect more than 15% of children each winter, often in sharp, community-wide outbreaks. Infection causes substantial injury to the upper and lower respiratory tract, with high fever, conjunctivitis, coryza, sore throat, and prominent cough. Complications occur frequently and include otitis media, sinusitis, croup, bronchitis, and bronchopneumonia. Rarer complications include febrile convulsions, encephalopathy, and Reye syndrome. The infection spreads readily among school children and household members. Complications requiring medical care or hospitalization are more likely to occur in children younger than 2 years and in those of any age with debilitating lung or heart disease or other chronic conditions (18,19).
Immunization is recommended for children at increased risk of complications.
Vaccine Preparations
Currently licensed vaccines for children include either inactivated, nonreplicating split influenza viruses or live attenuated influenza virus vaccines. For inactivated vaccines,
strains are grown in embryonated hen’s eggs, then partially purified, inactivated with formalin, and disrupted with detergents. Formulations are typically trivalent, containing the three strains anticipated to spread during the following influenza season. Formulations are updated annually to match changes in circulating strains. Recent formulations have contained an influenza B strain and two influenza A strains (H3N2, H1N1). Full-dose formulations contain 15 μg of hemagglutinin antigen from each strain per 0.5-mL dose. Nonvaccine components in the vaccines may include residual egg proteins, traces of formalin, and the detergent splitting agent and thimerosal preservative, gelatin, and neomycin.
strains are grown in embryonated hen’s eggs, then partially purified, inactivated with formalin, and disrupted with detergents. Formulations are typically trivalent, containing the three strains anticipated to spread during the following influenza season. Formulations are updated annually to match changes in circulating strains. Recent formulations have contained an influenza B strain and two influenza A strains (H3N2, H1N1). Full-dose formulations contain 15 μg of hemagglutinin antigen from each strain per 0.5-mL dose. Nonvaccine components in the vaccines may include residual egg proteins, traces of formalin, and the detergent splitting agent and thimerosal preservative, gelatin, and neomycin.
Inactivated vaccines are injected intramuscularly. They should be stored at 2#65518;C to 8#65518;C. A live, attenuated, trivalent vaccine (FluMist) for intranasal administration was recently licensed in the United States for use in healthy persons 2 to 49 years of age.
Available Products
Product: Fluarix
Manufacturer: GlaxoSmithKline
Year Licensed: 2005
Product: FluMist
Manufacturer: MedImmune Vaccines
Year Licensed: 2003
Product: Fluvirin
Manufacturer: Chiron Corporation
Year Licensed: 1988
Product: Fluzone
Manufacturer: Aventis Pasteur
Year Licensed: 1978
Beginning in 2007, the FDA approved a formulation of FluMist that is stable on storage by refrigeration. FluMist and Fluarix do not contain thimerosal, and both Fluvirin and Fluzone are available with reduced thimerosal formulation. For information on the thimerosal content in these vaccines, visit the FDA Web site (www.fda.gov).
Vaccine Effectiveness
Each year, the influenza vaccine contains three virus strains, representing the influenza viruses thought to be most likely to circulate in the United States in the upcoming winter.
When the match between the virus strains in the vaccine and the circulating viruses is close, the vaccine prevents illness in up to 90% of healthy adults younger than 65 years. Among elderly people, the vaccine is about 30% to 70% effective in preventing disease, but it is 50% to 60% effective in preventing hospitalization and 80% effective in preventing influenza-related death in the elderly.
The vaccine protects between 45% and 90% of healthy children from getting influenza. Studies indicate that the older and healthier children, who have received the influenza vaccine, are more likely to be protected. Influenza vaccination has also been shown to decrease middle ear infections among young children by about 30%.
One goal for widespread influenza vaccine utilization in the United States is to decrease the transmission of influenza viruses. Epidemiologic studies have demonstrated that children have the highest rates of influenza virus infections, suggesting that universal immunization of children could result in transmission of these viruses within communities from persons providing care or who are household contacts of people who are high risk for complications form influenza (including young infants younger than 6 months for whom there is no effective vaccine and no licensed treatment), those with chronic diseases, and those who are older than 50 years (especially those who are older than 65 years) (19).
Of particular concern are health care workers who also commonly acquire influenza virus infections and who have transmitted influenza viruses in hospitals and long-term care facilities. Vaccination of health care workers has been associated with decreased deaths among nursing home residents, for example.
Dose Schedule
Trivalent inactivated vaccine (TIV) is given by the intramuscular route and live attenuated influenza vaccine (LAIV) is administered as a nasal spray (see Table 57.1). The formulations in multidose vials contain thimerosal as a preservative.
When young children (6 months until 9 years of age) are vaccinated against influenza for the first time, they should receive two doses of age-appropriate influenza vaccine given 1 month apart. Those children who received only one dose in their first year of vaccination should receive two doses in the following year. Two doses administered 4 weeks apart are also recommended for children 2 to 8 years of age who are receiving LAIV for the first time. For children 6 to 35 months of age, a half dose (0.25 mL) of TIV (injected) is recommended, in contrast with 0.5 mL which is the usual dose for everyone older than 3 years (see Tables 57.2 to 57.4) (11,19).
In the United States, Fluzone (Aventis Pasteur) may be administered to children as young as 6 months of age. Fluvirin (Novartis Corp.) should be given only to children 4 years of age and older because its efficacy in younger people has not been demonstrated. Fluarix, Flulaval, and Afluria are not licensed for use in children but are licensed for adults 18 years of age and older. LAIV (Flumist) should be given only to healthy children and adolescents, aged 2 to 17 years, and healthy adults, aged 18 to 49 years (2).
Children 9 years of age or older should receive one dose of influenza vaccine each year. Ideally, all persons in the United States should receive the influenza vaccine at the beginning of October through November each year, prior to the influenza season, which generally peaks during late December through early March. However, vaccination later in the season is considered worthwhile.
Influenza vaccine is recommended for all children older than 6 months, all adults older than 50 years, and all persons who work in the health care industry should be immunized annually. In addition, those at increased risk of developing complications from influenza should be immunized, including women who will be pregnant during the influenza season (November to April).
Influenza vaccine is recommended for all contacts of children younger than 5 years, including women who are
breast-feeding. Women who are breast-feeding may receive either TIV or LAIV (unless LAIV is contraindicated because of other medical conditions). Vaccines should also be considered for those who can transmit influenza viruses to those at high risk for complications, including members of households with high-risk persons, including households that will include children younger than 6 months, persons coming in contact with children younger than 6 months, and persons who will come into contact with persons who live in nursing homes or other long-term care facilities.
breast-feeding. Women who are breast-feeding may receive either TIV or LAIV (unless LAIV is contraindicated because of other medical conditions). Vaccines should also be considered for those who can transmit influenza viruses to those at high risk for complications, including members of households with high-risk persons, including households that will include children younger than 6 months, persons coming in contact with children younger than 6 months, and persons who will come into contact with persons who live in nursing homes or other long-term care facilities.
In addition, influenza vaccine is recommended for children with long-term disorders of the lungs, heart, or circulation (including asthma or cystic fibrosis); metabolic diseases (including diabetes); kidney disorders; blood disorders (including anemia or sickle-cell disease); impaired immune systems (including immunosuppression caused by medications, malignancies, organ transplant, or human immunodeficiency virus [HIV] infection); and children who receive long-term aspirin therapy (and therefore have a higher chance of developing Reye syndrome if infected with influenza).
Influenza vaccine is encouraged for healthy people 6 years or older who plan to travel to foreign countries and areas where flu outbreaks may be occurring, such as the Tropics and the Southern Hemisphere from April through September; travel as part of large organized tourist groups that may include persons from areas of the world where influenza viruses are circulating; attend school or college and reside in dormitories or institutional settings; and wish to reduce their risk of becoming ill with influenza.
LAIV is not recommended for children younger than 2 years and adults older than 49 years, because safe use in these age groups has not been established; children and adolescents (2 to 17 years of age) receiving aspirin or aspirin-containing medications, because of the complications associated with aspirin and wild-type influenza virus infections in this age group; subjects with a history of asthma or other reactive airway diseases; persons with chronic underlying medical conditions that may predispose them to severe influenza infections; pregnant women; and subjects with a history of GBS.
LAIV is also not recommended for subjects who have had an anaphylactic reaction (allergic reactions that cause difficulty in breathing, which is often followed by shock) to eggs, egg products, or other components of the flu vaccine. There are antiviral agents which physicians can prescribe as an alternative for preventing influenza in such people (2,11). Children younger than 4 years should not receive Fluvirin 1 (Novartis International AG) as it has not been proven effective for this age group. LAIV should also not be given concurrently with other live-virus vaccines.
Adverse Effects
The majority of those immunized with TIV will have no adverse reactions. Of those who do have a side effect, most will have soreness or tenderness at the injection site. Fewer than 1% of adults immunized will also experience fever, chills, or a general sense of feeling unwell that lasts for 1 to 2 days. Children are more likely to experience these symptoms.
In very rare cases (far less than 1 out of 10,000) serious reactions can occur. Subjects who have an allergy to eggs (which are used in making the vaccine) or any component of the vaccine are at greater risk for a serious allergic reaction.
Because of an increase in the frequency of GBS (a progressive disorder affecting the nervous system) associated with the 1976 swine flu vaccine, subsequent flu vaccines have been closely monitored (20). It has been estimated that about one case of GBS may occur per million persons immunized with TIV, although these cases may not be related to TIV. Persons not at high risk for developing complications from influenza, who have developed GBS within 6 weeks of a previous influenza shot, should avoid subsequent influenza shots. If the risk from influenza is high, they should be vaccinated with an age-appropriate inactivated influenza vaccine because the established benefits of the vaccine justify vaccination. LAIV should not be given to individuals who have a history of GBS because safety in those persons has not been investigated.
Influenza vaccines given as a nasal spray are being used for adults in Russia and have been under development in the United States since the 1960s. LAIV administered as a nasal spray was approved by the FDA in June of 2003. It is the first nasally administered vaccine to be marketed in the United States and the first live virus influenza vaccine approved in the United States. The possible advantages of this type of vaccine are that it is easy to administer, has the potential to induce a broad mucosal and systemic immune response, and has been shown to be approximately 87% effective in children. Although TIV and LAIV appear to have similar effectiveness and safety profiles, no study has directly compared the efficacy or effectiveness of TIV and trivalent LAIV (18).
When there is a major change in the influenza virus strain from one year to the next, epidemics or pandemics (world outbreaks) can occur. During the 20th century, there were four major pandemics; the worst caused 21 million deaths worldwide and 500,000 deaths in the United States from 1918 to 1919. Between 1957 and 1986 there were 19 different flu epidemics in the United States; several of the most recent caused more than 40,000 deaths.
Japanese Encephalitis Vaccine
Japanese encephalitis (JE), a mosquito-borne arboviral (Flavivirus) infection, is the leading cause of viral encephalitis in Asia (2,21). Infection leads to overt encephalitis in 1 in 20 to 1,000 infected cases. Encephalitis usually is severe, resulting in a fatal outcome in 25% of cases and residual neuropsychiatric sequelae in 50% of cases. JE acquired during the first or second trimester of pregnancy may cause intrauterine infection and miscarriage. Infections that occur during the third trimester of pregnancy have not been associated with adverse outcomes in newborns. The virus is transmitted in an enzootic cycle among mosquitoes and vertebrate amplifying hosts, chiefly domestic pigs and, in some areas, wild Ardeid (wading) birds. Viral infection rates in mosquitoes range from less than 1% to 3%. These species are prolific in rural areas where their larvae breed in ground pools and flooded rice fields.
Thus, all elements of the transmission cycle are prevalent in rural areas of Asia and human infections occur principally in this setting. Because vertebrate amplifying hosts and agricultural activities may be situated within and at the periphery of cities, human cases occasionally are reported from urban locations.
Thus, all elements of the transmission cycle are prevalent in rural areas of Asia and human infections occur principally in this setting. Because vertebrate amplifying hosts and agricultural activities may be situated within and at the periphery of cities, human cases occasionally are reported from urban locations.
Vaccine Preparation
JE-VAX, Japanese Encephalitis Virus Vaccine Inactivated, is a sterile, lyophilized vaccine for subcutaneous use prepared by inoculating mice intracerebrally with JE virus, “Nakayama–NIH” strain, manufactured by The Research Foundation for Microbial Diseases of Osaka University (BIKEN). Infected brains are harvested and homogenized in phosphate buffered saline, pH 8.0. The homogenate is centrifuged and the supernatant inactivated with formaldehyde and then processed to yield a partially purified, inactivated virus suspension. This is further purified by ultracentrifugation through 40% w/v sucrose. The suspension is then lyophilized in final containers and sealed under dry nitrogen atmosphere. Thimerosal is added as a preservative to a final concentration of 0.007%. The diluent, which is sterile water, contains no preservative. Each 1.0 mL dose contains approximately 500 μg of gelatin, less than 100 μg of formaldehyde, less than 0.0007% v/v Polysorbate 80, and less than 50 ng of mouse serum protein. No myelin basic protein can be detected at the detection threshold of the assay (<2 ng per mL). Prior to reconstitution, the vaccine is a white caked powder, and after reconstitution the vaccine is a colorless transparent liquid (21).
Vaccine Usage
JE-VAX is indicated for active immunization against JE for persons 1 year of age and older. JE-VAX should be considered for use in persons who plan to reside in or travel to areas where JE is endemic or epidemic during a transmission season. JE-VAX is NOT recommended for all persons traveling to or residing in Asia. The incidence of JE in the location of intended stay, the conditions of housing, nature of activities, duration of stay, and the possibility of unexpected travel to high-risk areas are the factors that should be considered in the decision to administer vaccine. In general, vaccination should be considered for persons spending a month or longer in epidemic or endemic areas during the transmission season, especially if travel will include rural areas. Depending on the epidemic circumstances, vaccine should be considered for persons traveling less than 30 days whose activities, such as extensive outdoor activities in rural areas, place them at particularly high risk for exposure (21).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree