 Maternal Mortality and Morbidity
Maternal Mortality and Morbidity
To provide accurate informed consent, understanding both maternal and neonatal risks and benefits with surgery is essential. As a broad overview, cesarean delivery has higher maternal surgical risks for the current and subsequent pregnancies. This is balanced against lower rates of perineal injury and short-term pelvic floor disorders, described in Chapter 27 (p. 536). For the neonate, cesarean delivery offers lower rates of birth trauma and stillbirth. Conversely, rates of initial respiratory difficulties are greater with cesarean delivery.
For the mother, death attributable solely to cesarean delivery is rare in the United States. Even so, numerous studies attest to increased mortality risks. Clark and colleagues (2008), in a review of nearly 1.5 million pregnancies, found maternal mortality rates of 2.2 per 100,000 cesarean deliveries compared with 0.2 per 100,000 vaginal births. In a metaanalysis of 203 studies, Guise and coworkers (2010) reported a maternal mortality rate of 13 per 100,000 with elective repeat cesarean delivery compared with 4 per 100,000 women undergoing a trial of labor during VBAC attempt.
Similar to mortality rates, the frequency of some maternal complications is increased with all cesarean compared with vaginal deliveries (Table 30-2). Villar and associates (2007) reported that maternal morbidity rates increased twofold with cesarean compared with vaginal delivery. Principal among these are infection, hemorrhage, and thromboembolism. In addition, anesthetic complications, which also rarely include death, have a greater incidence with cesarean compared with vaginal delivery (Cheesman, 2009; Hawkins, 2011). Adjacent organs may be injured. The bladder laceration rate is 1 to 3 per 1000 cesarean deliveries, whereas that for ureteral injury approximates 0.3 per 1000 cases (Güngördük, 2010; Phipps, 2005; Rajasekar, 1997). Bowel damage occurs in approximately 1 in 1000 cesarean deliveries (Silver, 2006).
TABLE 30-2. Complications Associated with Low-Risk Planned Cesarean Deliverya Compared with Planned Vaginal Delivery among Healthy Women in Canada, 1991–2005
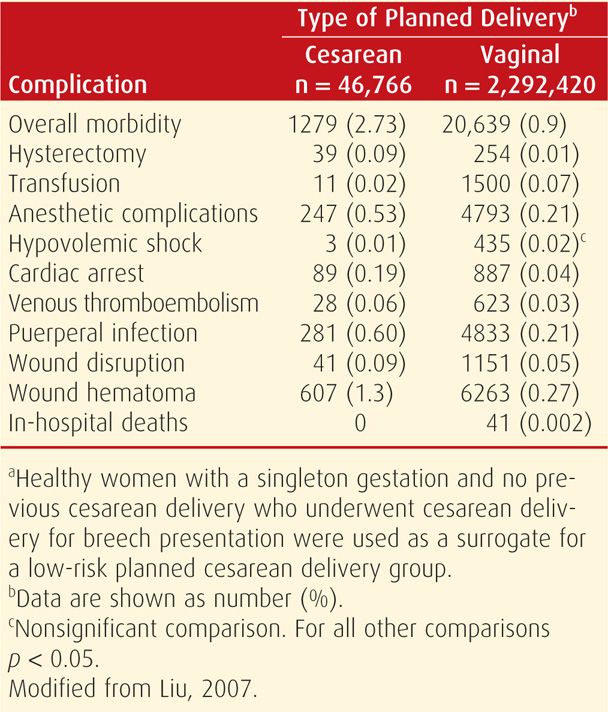
Women expressing a desire for elective primary cesarean delivery may be counseled that surgery offers decreased risks for hemorrhage and chorioamnionitis compared with planned primary vaginal birth. But this is again balanced against higher maternal rates of thromboembolism, hysterectomy, and rehospitalization for infection or wound complications; longer initial hospital stays; and greater rates of uterine rupture or abnormal placental implantation in subsequent pregnancies (Declercq, 2007; Geller, 2010; Liu, 2007).
Women who undergo a cesarean delivery are much more likely to be delivered by a repeat operation in subsequent pregnancies. For women undergoing subsequent cesarean, the maternal risks just described are even greater (Cahill, 2006; Marshall, 2011; Silver, 2006).
As an advantage, there is evidence that cesarean delivery is associated with lower rates of urinary incontinence and pelvic organ prolapse (Glazener, 2013; Gyhagen, 2013; Handa, 2011; Leijonhufvud, 2011; Rortveit, 2003). This protective advantage may persist to some degree over time, but cesarean delivery is not wholly protective (Dolan, 2010; MacArthur, 2011; Rortveit, 2001). Finally, cesarean delivery is not protective long-term for fecal incontinence (MacArthur, 2011, 2013; Nelson, 2010).
 Neonatal Morbidity
Neonatal Morbidity
Cesarean delivery is associated with less risk of fetal trauma. And this in many instances influences the choice of cesarean delivery despite the associated maternal risks. Alexander and colleagues (2006) found that fetal injury complicated 1 percent of cesarean deliveries. Skin laceration was most common, but others included cephalohematoma, clavicular fracture, brachial plexopathy, skull fracture, and facial nerve palsy. Cesarean deliveries following a failed operative vaginal delivery attempt had the highest injury rate, whereas the lowest rate—0.5 percent—occurred in the elective cesarean delivery group. That said, Worley and colleagues (2009) found that approximately a third of pregnant women who were delivered at Parkland Hospital entered spontaneous labor at term, and 96 percent of these delivered vaginally without adverse neonatal outcomes.
Although physical injury risks are lower, cesarean delivery per se may have no bearing on the neurodevelopmental prognosis of the infant. Scheller and Nelson (1994) in a report from the National Institutes of Health and Lien and associates (1995) presented data specifically refuting any association between cesarean delivery and lower rates of either cerebral palsy or seizures. Moreover, as discussed in Chapter 33 (p. 638), the incidences of neonatal seizures or cerebral palsy have not diminished as the rate of cesarean delivery has increased (Miller, 2008; Miller, 2013).
 Patient Choice in Cesarean Delivery
Patient Choice in Cesarean Delivery
As women have taken a more active role in their obstetrical care, some request elective cesarean delivery. Data regarding the true incidence of cesarean delivery on maternal request (CDMR) are limited, however, estimates are a 1- to 7-percent rate in the United States in 2003 (Gossman, 2006; Menacker, 2006).
Reasons for requested cesarean delivery include reduced risk of fetal injury, avoidance of the uncertainty and pain of labor, protection of pelvic floor support, and convenience. Thus, the debate surrounding CDMR includes its medical rationale from both a maternal and fetal-neonatal standpoint, the concept of informed free choice by the woman, and the autonomy of the physician in offering this choice.
To address this, the National Institutes of Health (2006) held a State-of-the-Science Conference on Cesarean Delivery on Maternal Request. A panel of experts critically reviewed available literature to form recommendations based on identified risks and benefits. It is noteworthy that most of the maternal and neonatal outcomes examined had insufficient data to permit such recommendations. Indeed, one of the main conclusions of the conference was that more high-quality research is needed to fully evaluate the issues. The American College of Obstetricians and Gynecologists (2013) concluded that data comparing planned cesarean and planned vaginal delivery were minimal and thus should be interpreted cautiously.
The panel was able to draw a few conclusions from existing information. Cesarean delivery on maternal request should not be performed before 39 weeks’ gestation unless there is evidence of fetal lung maturity. It should be avoided in women desiring several children because of the risk of placental implantation abnormalities and cesarean hysterectomy. Finally, it should not be motivated by the unavailability of effective pain management.
PATIENT PREPARATION
 Delivery Availability
Delivery Availability
There is no nationally recognized standard of care that codifies an acceptable time interval to begin performance of a cesarean delivery. Previously, a 30-minute decision-to-incision interval was recommended. In most instances, however, operative delivery is not necessary within this 30-minute time frame. Bloom and coworkers (2001) reported for the Maternal-Fetal Medicine Units Network that 69 percent of 7450 cesareans performed in labor commenced more than 30 minutes after the decision to operate. In a second study, Bloom and colleagues (2006) evaluated cesarean deliveries performed for emergency indications. They reported that failure to achieve a cesarean delivery decision-to-incision time of less than 30 minutes was not associated with a negative neonatal outcome. On the other hand, when faced with an acute, catastrophic deterioration in fetal condition, cesarean delivery usually is indicated as rapidly as possible, and thus purposeful delays are inappropriate. The American Academy of Pediatrics and the American College of Obstetricians and Gynecologists (2012) recommend that facilities giving obstetrical care should have the ability to initiate cesarean delivery in a time frame that best incorporates maternal and fetal risks and benefits.
 Informed Consent
Informed Consent
Obtaining informed consent is a process and not merely a medical record document (American College of Obstetricians and Gynecologists, 2012a). This conversation between a clinician and patient should enhance a woman’s awareness of her diagnosis and contain a discussion of medical and surgical care alternatives, procedure goals and limitations, and surgical risks. For women with a prior cesarean delivery, the option of a trial of labor should be included for suitable candidates. Also, in those desiring permanent sterilization, consenting for tubal ligation, as described in Chapter 39 (p. 720), can be completed at this time.
 Timing of Scheduled Cesarean Delivery
Timing of Scheduled Cesarean Delivery
Adverse neonatal sequelae from neonatal immaturity with elective delivery before 39 completed weeks are appreciable (Clark, 2009; Tita, 2009a). To avoid these, assurance of fetal maturity before scheduled elective surgery is essential as outlined by the American Academy of Pediatrics and the American College of Obstetricians and Gynecologists (2012) and discussed in Chapter 31 (p. 615). To assist with this and other components of cesarean delivery planning, the American College of Obstetricians and Gynecologists (2011a,c) has created Patient Safety Checklists to be completed before the planned surgery.
 Perioperative Care
Perioperative Care
If cesarean delivery is scheduled, a sedative may be given at bedtime the night before surgery. In general, no other sedatives, narcotics, or tranquilizers are administered until after the fetus is born. Oral intake is stopped at least 8 hours before the procedure. The woman scheduled for repeat cesarean delivery typically is admitted the day of surgery and evaluated by the obstetrical and anesthesia teams. Recently performed hematocrit and indirect Coombs test are reviewed, and if the latter is positive, then availability of compatible blood must be ensured.
As discussed in Chapter 25 (p. 509), regional analgesia is preferred for cesarean delivery. An antacid is given shortly before regional analgesia or induction with general anesthesia. One example is Bicitra, 30 mL orally in a single dose. This minimizes the lung injury risk from gastric acid aspiration. Once the woman is supine, a wedge beneath the right hip creates a left lateral tilt to aid venous return and avoid hypotension. According to the American College of Obstetricians and Gynecologists (2010), there are insufficient data to determine the value of fetal monitoring before scheduled cesarean delivery in women without risk factors. That said, fetal heart sounds should be documented in the operating room prior to surgery.
For further preparation, if hair obscures the operative field it should be removed the day of surgery by clipping. This is associated with fewer surgical site infections compared with shaving (Tanner, 2011). An indwelling bladder catheter is typically placed at Parkland Hospital to collapse the bladder away from the hysterotomy incision, to avert urinary retention secondary to regional analgesia, and to allow accurate postoperative urine measurement. Small studies support the nonuse of catheterization in hemodynamically stable women to minimize urinary infections (Li, 2011; Nasr, 2009).
The risk of thromboembolism is increased with pregnancy and almost doubled in those undergoing cesarean delivery (James, 2006). For this reason, for all women not already receiving thromboprophylaxis, the American College of Obstetricians and Gynecologists (2011d) recommends initiation of pneumatic compression hose before cesarean delivery. These are usually discontinued once the woman ambulates. In contrast, we favor the recommendations of American College of Chest Physicians for early ambulation for women without risk factors who are undergoing cesarean delivery (Bates, 2012). For women already receiving prophylaxis or those with increasing risk factors, prophylaxis is escalated as discussed in Chapter 52 and shown in Table 52-8 (p. 1046).
Some women scheduled for cesarean delivery have concurrent comorbidity that requires specific management in anticipation of surgery. Among others, these include insulin-requiring or gestational diabetes, coagulopathy or thrombophilia, chronic corticosteroid use, and significant reactive airway disease. Preparations for surgery in these women are discussed in the respective chapters covering these topics.
 Infection Prevention
Infection Prevention
Febrile morbidity is frequent after cesarean delivery. Numerous good-quality trials have proved that that a single dose of an antimicrobial agent given at the time of cesarean delivery significantly decreases infection morbidity. Although more obvious for women undergoing unscheduled cesarean delivery, this practice also significantly lowers the postoperative infection rate in women undergoing elective surgery (American College of Obstetricians and Gynecologists, 2011b). Depending on drug allergies, most recommend a single intravenous dose of a β-lactam antimicrobial—either a cephalosporin or extended-spectrum penicillin derivative. A 1-g dose of cefazolin is an efficacious and cost-effective choice. For obese women, a 2-g dose usually provides adequate coverage, although Pevzner and associates (2011) showed this dose may be inadequate for those with body mass index > 40. In women with significant penicillin or cephalosporin allergy, a single 600-mg intravenous dose of clindamycin combined with a weight-based dose of aminoglycoside is an alternative. A 900-mg clindamycin dose is used for obese patients.
Antimicrobial administration before surgical incision has been shown to lower postoperative infection rates without adverse neonatal effects compared with drug administration after umbilical cord clamping (Sullivan, 2007; Tita, 2009b; Witt, 2011). For this reason, the American College of Obstetricians and Gynecologists (2011b) recommends that prophylaxis be administered within the 60 minutes prior to the start of planned cesarean delivery. For emergent delivery, prophylaxis should be given as soon as feasible.
Preoperative preparation of the abdominal wall skin is effective to prevent wound infection. Either chlorhexidine or povidone-iodine solutions can be used. In addition, to prevent postoperative metritis following cesarean delivery, preoperative vaginal cleansing with a povidone-iodine scrub has been evaluated in small randomized trials (Haas, 2013). Some show benefit, whereas others do not (Reid, 2001; Starr, 2005; Yildirim, 2012). Vaginal cleansing is not a part of preoperative preparation at Parkland Hospital.
Antibiotic prophylaxis against infective endocarditis is not recommended for most with cardiac conditions—exceptions are women with cyanotic heart disease, prosthetic valves, or both (American College of Obstetricians and Gynecologists, 2011b). Regimens selected for routine cesarean infection prophylaxis will also serve as appropriate endocarditis coverage (Chap. 49, p. 991).
 Surgical Safety
Surgical Safety
The Joint Commission (2013) established a protocol to prevent surgical errors that encompasses three components: (1) preprocedure verification of all relevant documents, (2) marking the operative site, and (3) completion of a “time out” before procedure initiation. The “time out” requires attention of the entire team to confirm that the patient, site, and procedure are correct. Important discussions also include introduction of the patient-care team members, verification of prophylactic antibiotics, estimation of procedure length, and communication of anticipated complications. Additionally, requests for special instrumentation should be addressed preoperatively to prevent potential patient compromise and intraoperative delays.
An instrument, sponge, and needle count before and after surgery and vaginal delivery is crucial to surgical safety. If counts are not reconciled following abdominal or vaginal examination, then radiographic imaging for retained foreign objects is obtained (American College of Obstetricians and Gynecologists, 2012b).
TECHNIQUE FOR CESAREAN DELIVERY
With minor variations, surgical performance of cesarean delivery is comparable worldwide. Most steps are founded on evidence-based data, and these have been reviewed by Dahlke (2013) and Hofmeyr (2009) and their associates. As with all surgery, a clear understanding of relevant anatomy is essential, and this is described and illustrated in Chapter 2 (p. 16).
 Abdominal Incision
Abdominal Incision
In obstetrics, usually a midline vertical or a suprapubic transverse incision is chosen for laparotomy. Transverse abdominal entry is by either Pfannenstiel or Maylard incisions. Of these, the Pfannenstiel incision is selected most frequently for cesarean delivery. Transverse incisions follow Langer lines of skin tension, and superior cosmetic results compared with vertical incisions can be achieved. Additionally, decreased rates of postoperative pain, fascial wound dehiscence, and incisional hernia compared with vertical entry are benefits. Use of the Pfannenstiel incision, however, is often discouraged for cases in which a large operating space is essential or in which access to the upper abdomen may be needed. Because of the layers created during incision of the internal and external oblique aponeuroses with transverse incisions, purulent fluid can collect between these. Therefore, cases with high infection risks may favor a midline incision. Last, neurovascular structures, which include the ilioinguinal and iliohypogastric nerves and superficial and inferior epigastric vessels, are often encountered with transverse incisions. Logically, bleeding, wound hematoma, and neurological disruption may more frequently complicate these incisions compared with vertical incision. With repeat cesarean delivery, reentry through a Pfannenstiel incision usually is more time consuming and difficult because of scarring.
The Maylard incision differs mainly from the Pfannenstiel in that the bellies of the rectus abdominis muscles are transected horizontally to widen the operating space. It is technically more difficult due to its required isolation and ligation of the inferior epigastric arteries, which lie lateral to these muscle bellies.
Vertical infraumbilical incisions provide quick entry to shorten incision-to-delivery time (Wylie, 2010). Moreover, this incision has minimal blood loss, superior access to the upper abdomen, generous operating room, and the flexibility for easy wound extension if greater space or access is needed. No important neurovascular structures traverse this incision, and aponeuroses at the linea alba are fused. Main disadvantages are poorer cosmetic results, higher fascial dehiscence or incisional hernia rates, and greater postoperative pain. For morbidly obese patients, a vertical incision that extends up and around the umbilicus may be preferable to avoid cutting through a large pannus (Fig. 48-7, p. 968).
Transverse Incisions
With the Pfannenstiel incision, the skin and subcutaneous tissue are incised using a low, transverse, slightly curvilinear incision. This is made at the level of the pubic hairline, which is typically 3 cm above the superior border of the symphysis pubis. The incision is extended somewhat beyond the lateral borders of the rectus abdominis muscles. It should be of adequate width to accommodate delivery—12 to 15 cm is typical.
Sharp dissection is continued through the subcutaneous layer to the fascia. The superficial epigastric vessels can usually be identified halfway between the skin and fascia, several centimeters from the midline, and coagulated. If lacerated, these may be suture ligated with 3-0 plain gut suture or coagulated with an electrosurgical blade.
The fascia is then incised sharply at the midline. The anterior abdominal fascia is typically composed of two visible layers, the aponeurosis from the external oblique muscle and a fused layer containing aponeuroses of the internal oblique and transverse abdominis muscles. Ideally, the two layers are individually incised during lateral extension of the fascial incision. The inferior epigastric vessels typically lie outside the lateral border of the rectus abdominis muscle and beneath the fused aponeuroses of the internal oblique and transverse abdominis muscles. Thus, although infrequently required, extension of the fascial incision further laterally may cut these vessels. Therefore, if lateral extension is needed, these vessels should be identified and coagulated or ligated to prevent bleeding and vessel retraction.
Once the fascia is incised, the inferior fascial edge is grasped with suitable clamps and elevated by the assistant as the operator separates the fascial sheath from the underlying rectus muscles either bluntly or sharply until the superior border of the symphysis pubis is reached. Any blood vessels coursing between the sheath and muscles are clamped, cut, and ligated, or they are coagulated with an electrosurgery blade. Next, the superior fascial edge is grasped and again, separation of fascia from the rectus muscles is completed. Meticulous hemostasis is imperative to lower rates of infection and bleeding. The fascial separation is carried near enough to the umbilicus to permit an adequate midline longitudinal incision of the peritoneum. The rectus abdominis and pyramidalis muscles are then separated in the midline by sharp and blunt dissection to expose the transversalis fascia and peritoneum.
The transversalis fascia and preperitoneal fat are dissected carefully to reach the underlying peritoneum. The peritoneum near the upper end of the incision is opened carefully, either bluntly or by elevating it with two hemostats placed approximately 2 cm apart. The tented fold of peritoneum between the clamps is examined and palpated to ensure that omentum, bowel, or bladder is not adjacent. The peritoneum is then incised. The incision is extended superiorly to the upper pole of the incision and downward to just above the peritoneal reflection over the bladder. Importantly, in women who have had previous intraabdominal surgery, including cesarean delivery, omentum or bowel may be adhered to the undersurface of the peritoneum. Moreover, in women with obstructed labor, the bladder may be pushed cephalad almost to the level of the umbilicus.
Vertical Incision
An infraumbilical midline vertical incision begins 2 to 3 cm above the superior margin of the symphysis and should be of sufficient length to allow fetal delivery without difficulty. Therefore, its length should correspond with the estimated fetal size, and 12 to 15 cm is typical. Sharp or electrosurgical dissection is performed to the level of the anterior rectus sheath. A small opening is made sharply with scalpel in the upper half of the linea alba. Placement here avoids potential cystotomy. Index and middle fingers are placed beneath the fascia, and the fascial incision is extended superiorly and inferiorly with scissors or scalpel. Midline separation of the rectus muscles and pyramidalis muscles and peritoneal entry are then similar to those with the Pfannenstiel incision.
 Hysterotomy
Hysterotomy
Most often, the lower uterine segment is incised transversely as described by Kerr in 1921. Occasionally, a low-segment vertical incision as described by Krönig in 1912 may be used. The classical incision is a vertical incision into the body of the uterus above the lower uterine segment and reaches the uterine fundus. In practice, however, the classical incision is similar to the low-vertical incision, which is typically extended cephalad only to the extent required for fetal delivery. For most cesarean deliveries, the transverse incision is preferred. Compared with a classical incision, it is easier to repair, is located in the inactive segment and thus least likely to rupture during a subsequent pregnancy, causes less incision-site bleeding, and promotes less bowel or omentum adherence to the myometrial incision.
Low Transverse Cesarean Incision
Before any hysterotomy, the surgeon should palpate the fundus and adnexa to identify degrees of uterine rotation. The uterus may be dextrorotated so that the left round ligament is more anterior and closer to the midline. In such cases, hysterotomy placement is modified to keep the incision centered within the lower segment. This avoids extension into and laceration of the left uterine artery. With thick meconium or infected amnionic fluid, some surgeons prefer to put a moistened laparotomy sponge in each lateral peritoneal gutter to absorb fluid and blood that escape from the opened uterus. A moist sponge may also be used to pack protruding bowel away from the operative field.
The reflection of peritoneum above the upper margin of the bladder and overlying the anterior lower uterine segment—termed the bladder flap—is grasped in the midline with forceps and incised transversely with scissors (Fig. 30-1). Bladder flap creation effectively moves the bladder away from the planned hysterotomy site and prevents bladder laceration if an unintended inferior hysterotomy extension occurs during fetal delivery.
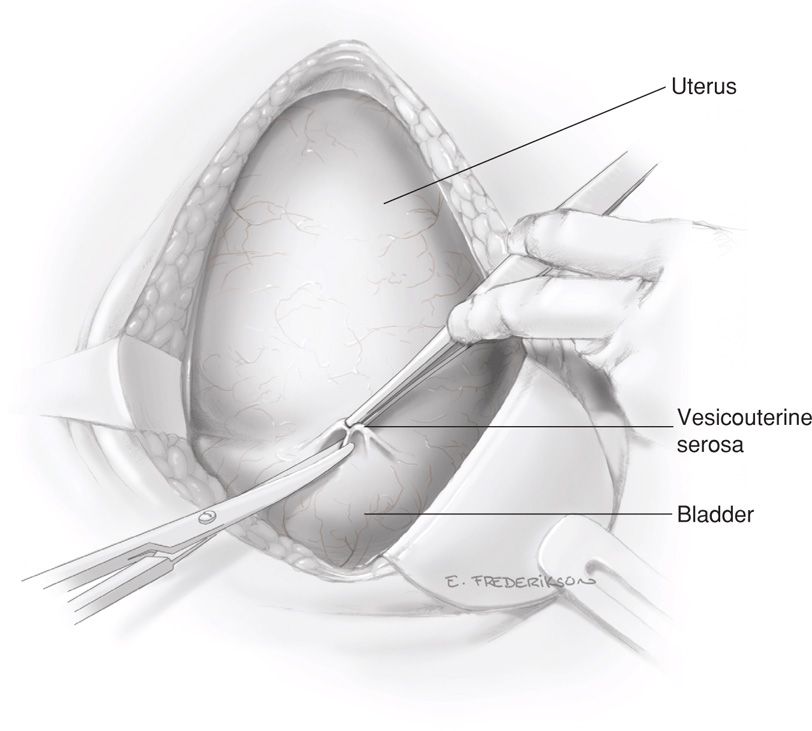
FIGURE 30-1 The loose vesicouterine serosa above the bladder reflection is grasped with forceps and incised with Metzenbaum scissors.
Following this initial incision, scissors are inserted between the vesicouterine serosa and myometrium of the lower uterine segment. The scissors are pushed laterally from the midline on each side to further open the visceral peritoneum and expose the myometrium. This transverse peritoneal incision extends almost the full length of the lower uterine segment. As the lateral margin on each side is approached, the scissors are directed somewhat more cephalad (Fig. 30-2). The lower edge of peritoneum is elevated, and the bladder is gently separated from the underlying myometrium with blunt or sharp dissection within this vesicouterine space (Fig. 30-3).
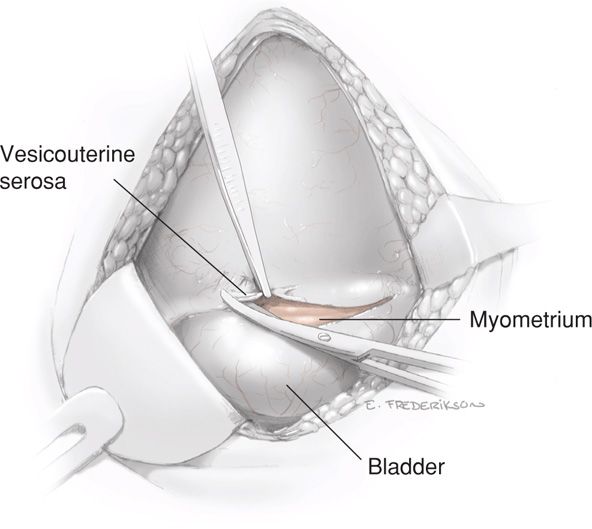
FIGURE 30-2 The loose serosa above the upper margin of the bladder is elevated and incised laterally.
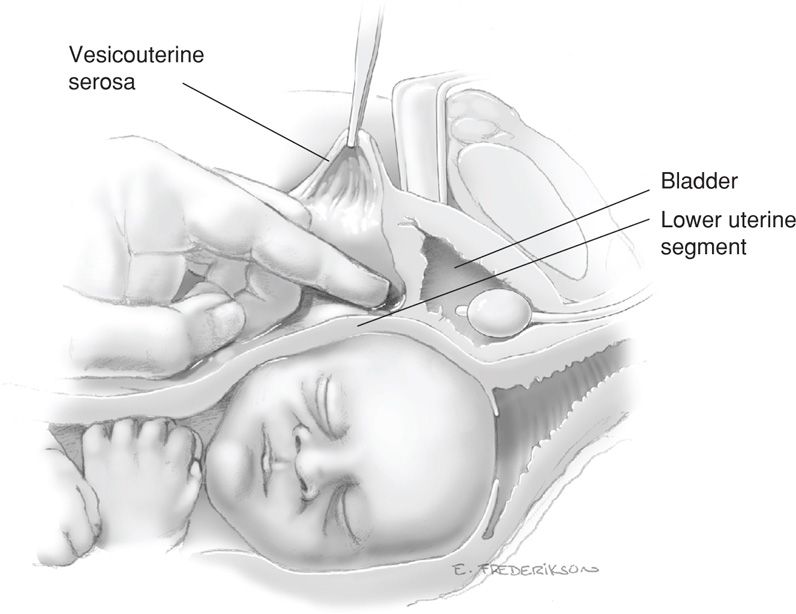
FIGURE 30-3 Cross section shows blunt dissection of the bladder off the uterus to expose the lower uterine segment.
In general, this caudad separation of bladder does not exceed 5 cm and usually is less. It is possible, especially with an effaced, dilated cervix, to dissect downward so caudally as to inadvertently expose and then enter the underlying vagina rather than the lower uterine segment. However, in instances in which cesarean hysterectomy is planned or anticipated, extended caudad bladder dissection is recommended to aid total hysterectomy and decrease the risk of cystotomy.
Some surgeons do not create a bladder flap. The main advantage is a shorter skin incision-to-delivery time, however, data supporting this practice are limited (Hohlagschwandtner, 2001; Tuuli, 2012).
The uterus is entered through the lower uterine segment approximately 1 cm below the upper margin of the peritoneal reflection. It is important to place the uterine incision relatively higher in women with advanced or complete cervical dilatation. Failure to adjust increases the risk of lateral extension of the incision into the uterine arteries. It may also lead to incision of the cervix or vagina rather than the lower uterine segment. Such incisions into the cervix can create significant postoperative distortion of cervical anatomy. Correct placement uses the vesicouterine serosal reflection as a guide.
Uterine Incision. The uterus can be incised by a variety of techniques. Each is initiated by using a scalpel to transversely incise the exposed lower uterine segment for 1 to 2 cm in the midline (Fig. 30-4). This must be done carefully to avoid fetal laceration. Careful blunt entry using hemostats or fingertip to split the muscle may be helpful. Once the uterus is opened, the incision can be extended by simply spreading the incision, using lateral and slightly upward pressure applied with each index finger (Fig. 30-5). Alternatively, if the lower uterine segment is thick, then cutting laterally and then slightly upward with bandage scissors will extend the incision. Importantly, when scissors are used, the index and midline fingers of the nondominant hand should be insinuated beneath the myometrium and above fetal parts to prevent fetal laceration. Comparing blunt and sharp extensions of the initial uterine incision, sharp extension is associated with an increased estimated blood loss, but postoperative hematocrit changes, need for transfusion, and infection rates are not different (Xu, 2013).
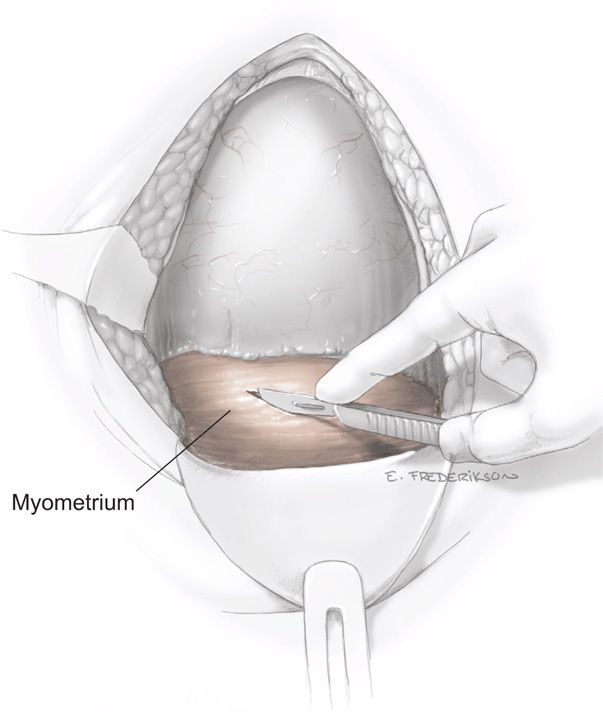
FIGURE 30-4 The myometrium is carefully incised to avoid cutting the fetal head.
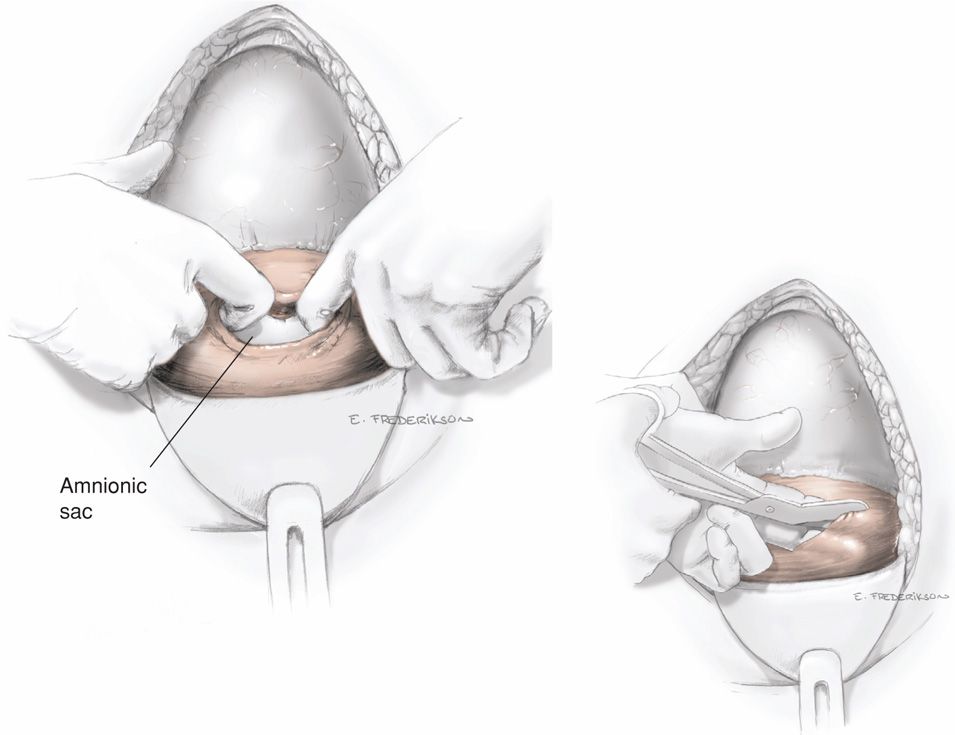
FIGURE 30-5 After entering the uterine cavity, the incision is extended laterally with fingers or with bandage scissors (inset).
The uterine incision should be made large enough to allow delivery of the head and trunk of the fetus without either tearing into or having to cut into the uterine vessels that course along the lateral uterine margins. If the placenta is encountered in the incision line, it must be either detached or incised. When the placenta is incised, fetal hemorrhage may be severe. Thus, delivery and cord clamping should be performed as soon as possible.
At times, a low-transverse hysterotomy is selected but provides inadequate room for delivery. In such instances, one corner of the hysterotomy incision is extended cephalad into the contractile portion of the myometrium—a J incision. If this is completed bilaterally, a U incision is formed. Last, some prefer to extend in the midline—a T incision. As expected, these have been linked with higher intraoperative blood loss (Boyle, 1996; Patterson, 2002). Moreover, as these extend into the contractile portion, a trial of labor and vaginal delivery are more likely contraindicated in future pregnancies.
Delivery of the Fetus. In a cephalic presentation, a hand is slipped into the uterine cavity between the symphysis and fetal head. The head is elevated gently with the fingers and palm through the incision. Once the head enters the incision, delivery may be aided by modest transabdominal fundal pressure (Fig. 30-6).
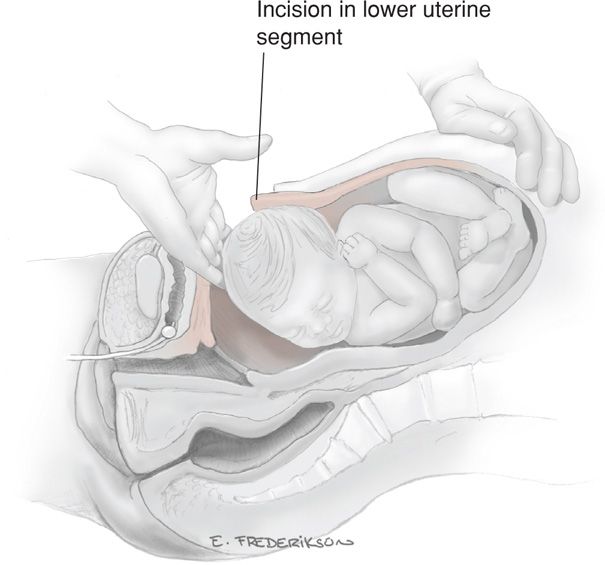
FIGURE 30-6 Delivery of the fetal head.
After a long labor with cephalopelvic disproportion, the fetal head may be tightly wedged in the birth canal. This situation can have disastrous results, and there are three considerations for delivery. First, a “push” method may be used. With this, upward pressure exerted by a hand in the vagina by an assistant will help to dislodge the head and allow its delivery above the symphysis. Relief of such head impaction increases the risk of hysterotomy extension and associated blood loss as well as fetal skull fracture. As an alternative, a “pull” method is used in which the fetal legs are grasped and delivered through the hysterotomy opening. The fetus is then delivered by traction as one would complete a breech extraction. Support for this latter approach comes only from small randomized trials and case series (Bastani, 2012; Shazly, 2013; Veisi, 2012). Last, a low vertical hysterotomy incision, which will give more room for the “pull” technique, may be selected. If a low transverse incision has already been made, then this can be extended to a J-, U-, or T-incision for room.
Conversely, in women without labor, the fetal head may be unmolded and without a leading cephalic point. The round head may be difficult to lift through the uterine incision in a relatively thick lower segment that is unattenuated by labor. In such instances, either forceps or a vacuum device may be used to deliver the fetal head as shown in Figure 30-7.
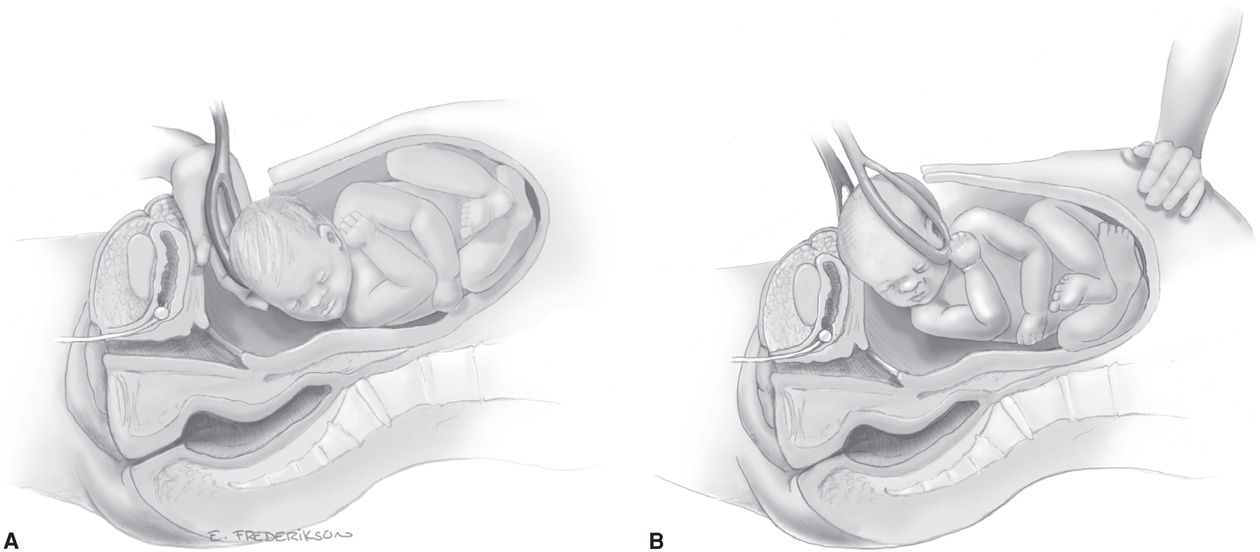
FIGURE 30-7 A. The first cesarean forceps blade is placed. B. Slight upward and outward traction is used to lift the head through the incision.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


