Cervical Ripening and Induction of Labor
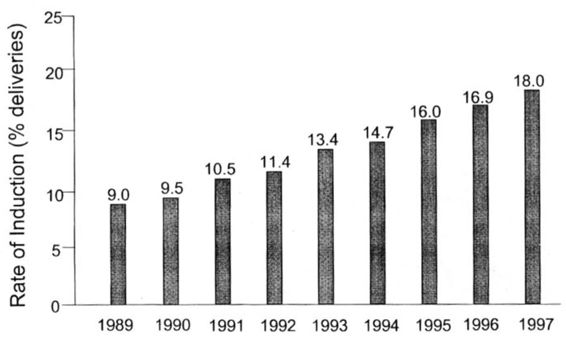
Induction of labor is a common procedure during pregnancy. According to the Centers for Disease Control and Prevention (CDC), rates for induction of labor have doubled from 9 percent in 1989 to 18 percent in 1997 (Ventura, 1999; Fig. 29-1). This increase has been noted both at community hospitals and at university tertiary care hospitals (Beebe, 2000). Mathews (1997) reported on trends in induction of labor between 1989 and 1995. Rates for induction of labor increased between 1989 and 1995 for both black and white women in all age categories. Women whose pregnancies extended beyond the expected gestation of 37 weeks consistently had much higher rates of induction. Declines in the cesarean section rate were greater for births that were induced than for those without this procedure.
FIGURE 29-1. Rates of pregnancies undergoing an induction of labor in the United States between 1989 and 1997.
Pregnancy termination is a voluntary option up to 23 weeks, 6 days in most states. Induction beyond this period must be based on either a fetal or maternal indication or both. Induction is indicated when the benefits to the mother and/or fetus outweigh those of continuing the pregnancy. Indications of induction of labor have essentially not changed. Table 29-1 lists conditions associated with measured maternal and fetal morbidity for which an induction of labor would be justified (American College of Obstetricians and Gynecologists, 1999). Primary indications include active medical disorders, such as diabetes or hypertension; postdatism; and prolonged ruptured membranes. When the fetus is at risk, indications for induction include oligohydramnios, altered growth, and intrauterine demise. A previous precipitate delivery and the patient living far from the hospital are logistic reasons to induce. Suspected fetal macrosomia alone is not an adequate reason to induce (American College of Obstetricians and Gynecologists, 1999).
TABLE 29-1. Indications and Contraindications to Cervical Ripening
Despite these well-known indications for labor induction, many inductions of labor are elective at term with no well-justified or well-documented reason (Beebe, 2000). This finding is especially common at community hospitals. Cesarean section rates are not necessarily higher in cases of presumed elective induction, as compared with waiting until spontaneous labor, if the cervix is favorable.
Documentation in the medical record about the reason for cervical ripening is recommended, although obtaining written informed consent is not absolutely necessary. In general, as long as there is no contraindication to labor, there should be no contraindication to using cervical ripening agents or uterine stimulating drugs.
Absolute contraindications to an induction are few, because certain clinical situations (eg, active herpes infection in the presence of fetal death) may still warrant induction of labor. Examples of conditions in which caution is recommended are multifetal gestation, polyhydramnios, maternal cardiac disease, grand multiparity, breech presentation, and fetal presenting part above the pelvic inlet. Although manufacturers report that a prior uterine incision is a contraindication to a cervical ripening agent, the physician has a right to prescribe medications for off label indications with proper patient awareness of risks and benefits. An amniocentesis, to test for fetal lung maturity, may be necessary when intervention is planned before term or when the gestational age is unknown (American College of Obstetricians and Gynecologists, 1991; Table 29-2).
TABLE 29-2. Criteria of Assumed Fetal Maturity
CERVICAL MATURATION DURING PREGNANCY
Approximately half of all patients undergoing an induction of labor have an unfavorable cervix. Cervical ripening refers to a prelabor change in the physical and biochemical configuration of collagen fiber in the uterine cervix, allowing for greater compliance during labor. In the past, the cervix was believed to play a passive role in parturition, with dilatation and effacement occurring only in response to uterine contractions. Cervical ripening is recognized, instead, as an active process in which the cervix changes shape and consistency. The cervix is considered to be ripe when it most closely assumes the characteristics of a term cervix that is about to enter spontaneous labor.
The development and maturation of the cervix and myometrium are regulated by the same factors and proceed together throughout gestation. Danforth and associates (1974) described the effect of pregnancy on the human cervix. The cervix is firm in the first trimester, with 50 percent of its dry weight consisting of tightly aligned and bound collagen and with 20 percent being smooth muscle. The remainder is ground substance produced by fibroblasts and composed of elastin and glycosaminoglycans (chondroitin, dermatan sulfates, and hyaluronidase). As pregnancy progresses, the collagen fibers become disrupted because concentrations of hyaluronidase that weakly binds to collagen increase from 6 to 33 percent, while concentrations of dermatan and chondroitin sulfates, which bind collagen more tightly, decrease. In addition, concentrations of collagenase and elastase enzymes increase, leading to a gradual breakdown in the structure of collagen and to a reduction of collagen content. The vascularity and water content of the cervix also increase with advancing gestation. These histologic changes are influenced by prostaglandins, estrogen/progesterone, relaxin, and leukotrienes.
Parity, cervical status, and station affect the duration of any induction of labor more than baseline uterine sensitivity to oxytocin does. In most pregnancies, some degree of cervical ripening precedes spontaneous labor. However, the cervix is often unripe or unfavorable at earlier gestational ages and in a significant number of postdate pregnancies.
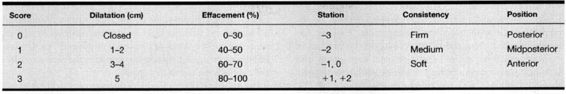
In 1964, Bishop and Edward described a scoring system for multiparous patients (Table 29-3) that considered dilatation, effacement, station, consistency, and position (Bishop, 1964). A cervical score exceeding 8 was predictive of a vaginal delivery, regardless of whether labor was induced or spontaneous. A technical bulletin of the American College of Obstetricians and Gynecologists (ACOG) later suggested that a cervical score of at least 6 was considered to be favorable and to likely result in successful labor induction without need for a cervical ripening agent (American College of Obstetricians and Gynecologists, 1991). Induction of labor with a lower cervical score is associated with a higher risk of a failed induction, prolonged labor, and cesarean delivery (Brindley and Sokol, 1988).
TABLE 29-3. Bishop Scoring System
CERVICAL RIPENING TECHNIQUES
Cervical ripening in the presence of an unfavorable cervix (long, firm, closed, or minimally dilated) can be undertaken using either mechanical or hormonal methods. As long as there is no contraindication to labor, there should be no contraindication to using either oxytocin or a ripening agent. Mechanical techniques include the intracervical insertion either of rubber catheters with balloons (ie, a single- or double-balloon 16- or 24-French Foley catheter) or of osmotic dilators. Nipple stimulation is possible by using a breast pump or by tactile stimulation for a few minutes on each nipple. Examples of hormonal or biochemical methods that have undergone clinical investigation for cervical ripening include prostaglandins (PG), RU 486 (mifepristone), estrogen, DHEAS, glyceryl trinitrate, and relaxin.
PROSTAGLANDINS
Prostaglandins consist of 20 carbon compounds synthesized in all cells (Craft, 1972; Ueland and Conrad, 1983). Exogenous prostaglandins have been administered orally, intravaginally, intracervically, or extra-amniotically to ripen the cervix and induce labor. Each of these routes has drawbacks. The application of prostaglandins in the upper vagina or in the cervical canal is more effective than a placebo with less total dose than when given systemically, thereby decreasing the total dose, maternal systemic effects, and uterine hyperstimulation (Brindley and Sokol, 1988). PGF2 is not available as a cervical ripening agent, because it requires 10 times the equivalent dose of PGE2 for equal efficacy and results in more systemic side effects.
Cervical changes described in physiologic cervical ripening and spontaneous labor are reproduced with exogenous prostaglandins. A dissolution of collagen, increase in glycosaminoglycans, and increase in fibroblast activity have been described in early gestation in humans and late pregnancy in rabbits (Danforth, 1974; Uldbjerg, 1981; Rayburn, 1994). Although these ripening effects may occur without contractions, prostaglandins are known to enhance myometrial sensitivity to oxytocin (Huszar and Walsh, 1991; Calder and Greer, 1991; Anderson and Turnbull, 1968; Wikland and coworkers, 1984; Gillespie, 1972). Prostaglandins have been reported to accelerate gap junction formation leading to more coordinated uterine contractions. Myometrial strips taken from the fundus during spontaneous labor have been reported to be stimulated with PGE2, while smooth muscle of the lower uterine segment and cervix are inhibited by PGE2 (Wikland and associates, 1984; Gillespie, 1972; Takahashi and colleagues, 1980; Liggins, 1978).
Maternal systemic effects occur very infrequently when low doses of prostaglandins are applied topically. Reported systemic effects include fever, vomiting, and diarrhea, all of which are very uncommon (usually <1 percent), dose dependent, mild, and reversible, either with removal of the preparation or with expectant management. Caution has been expressed about use of any prostaglandin product in cases of glaucoma, severe hepatic or renal impairment, and asthma. Bronchial constriction or significant blood pressure changes have not been reported after low doses of PGE2, however, and asthma is not a contraindication for its use. Increasing experience has been gained from use of low-dose prostaglandins after prior cesarean sections (Wing 1998; Raskin, 1999). Continuous monitoring is recommended among inpatients, and risk of a rupture must be explained beforehand.
Neonatal outcomes following prostaglandin cervical ripening compare favorably with those of oxytocin induction alone. Closure of the neonatal ductus arteriosus is not delayed (Danford and coworkers, 1993) despite low-dose intrapartum PGE2 therapy. Infant follow-up at 2 and 6 months of age has shown no unusual sequelae from the use of PGE2 gel.
MISOPROSTOL
Misoprostol (Cytotec, Searle, Chicago, IL) is an antiulcer medication containing a PGE1 analogue. It has gained widespread use for cervical ripening, despite lack of Food and Drug Administration (FDA) approval for this indication, because of its much lower cost. Each 100- or 200-μg tablet is stable at room temperature and easy to administer. Each tablet is usually divided into 25- or 50-μg doses, and then placed intravaginally every 3 or 4 hours. Any contractions are usually mild, but they may be sustained. Hyperstimulation of the uterus is dose dependent and occurs in 3–5 percent of cases (Wing, 1999).
Misoprostol is an effective agent for cervical ripening and labor induction when used judiciously and cautiously. Multiple trials have proved that misoprostol is an effective agent, as compared with oxytocin or prostaglandin E2, for cervical ripening and labor induction in term pregnancy (Sanchez-Ramos, 1993, 1998; Church, 1995; Wing, 1995,1998); however, investigations continue regarding the optimal dose, dosing regimen, and route of administration (Sanchez-Ramos, 1997; Wing, 1999).
In November 1999, the American College of Obstetricians and Gynecologists recommended the 25 μg dose, given vaginally every 3 hours (American College of Obstetricians and Gynecologists, 1999). Unlike trials where 50 μg of misoprostol was used, the 25 (μg dose administered intravaginally as frequently as every 3 hours was not associated with an increase in uterine tachysystole, hyperstimulation, or meconium passage when compared with PGE2. Further trials are required to (1) define an optimal dosing regimen for misoprostol and (2) confirm the safety of outpatient therapy.
Uterine contraction abnormalities are often found in association with higher misoprostol doses. Some trials also indicate increased frequencies of meconium passage, neonatal acidemia, and cesarean delivery for fetal distress in women receiving higher doses of misoprostol. Overall, most trials fail to demonstrate a significant change in the cesarean delivery rate with the use of this agent.
In August 2000, the medical director of Searle mailed to all obstetricians a warning that “misoprostol administration by any route is contraindicated in women who are pregnant because it can cause abortion. Cytotec is not approved for the induction of labor or abortion.”
CERVIDIL
Cervidil (Forest Laboratories, St. Louis, MO) is available as a 10-mg vaginal insert that releases PGE2 (dinoprostone) at 0.3 mg per hour during the recommended 12-hour dosing period. Uterine contractions are gradual and usually peak several hours after insertion of the medication (Smith, 1994). The risk of hyperstimulation is approximately 5–10 percent, making outpatient use inadvisable (Rayburn, 1992). Continuous fetal heart rate monitoring is recommended, although there is no evidence to support this (American College of Obstetricians and Gynecologists, 1999). A string attached to the insert allows for easy removal.
The controlled-release PGE2 intravaginal insert is associated with the initiation of cervical ripening and active labor during the 12-hour dosing period, frequently without the need for oxytocin (Witter, 1996). In this regard, approximately one-third of all women had the intravaginal insert removed before the end of the 12-hour dosing period because cervical ripening and active labor had been initiated (Blair, 1997). The cesarean section rate for induction failure following the administration of an intravaginal insert was very low (3.3–4.5 percent), and the vaginal delivery rate was similar to or greater than rates obtained with either placebo or multidose PGE2 gels.
Use of prostaglandins for the induction of labor has been scrutinized intensively as a means of reducing the economic costs associated with cesarean deliveries. As a consequence, the use of the controlled-release, intravaginal insert to induce labor, with a very-low induction failure rate, appears to be a beneficial therapeutic alternative. In addition, other indications for cesarean section, such as failure to progress and fetal intolerance to labor, are not associated with the efficacy of the intravaginal insert because these indications are normally related to a high-risk patient group in advanced labor.
PREPIDIL
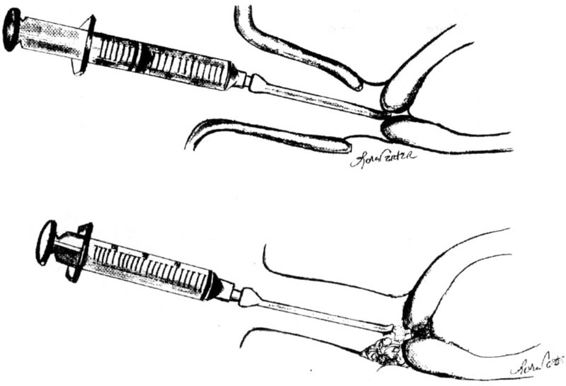
Prepidil (Pharmacia Upjohn, Kalamazoo, MI) is a 2.5-mL gel that contains 0.2 mg of PGE2 (dinoprostone). Figure 29-2 illustrates how the standard 2–3 mL volume is placed in the cervical canal up to the internal cervical os. A speculum is necessary to adequately visualize the external os for intracervical instillation. The cervix may be swabbed to remove any excess secretions beforehand. Caution should be taken not to administer the gel above the internal cervical os, because this increases the risk of uterine hyperstimulation. The patient is encouraged to remain supine for at least 30 minutes following gel installation to minimize leakage of the gel. Careful fetal heart rate monitoring is recommended for at least 30 minutes after instillation (American College of Obstetricians and Gynecologists, 1995). According to the manufacturer, at least 6 hours should elapse before either the dose is repeated or intravenous oxytocin is administered. More than a single dose is often necessary, and the manufacturer recommends a maximum of three doses during 24 hours.
FIGURE 29-2. The standard 2–3 mL volume is placed in the cervical canal at the level of the internal cervical os (top) or, alternatively, at the posterior vaginal fornix next to the external cervical os (bottom).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree