Three-year-old child with spastic quadriplegic CP.
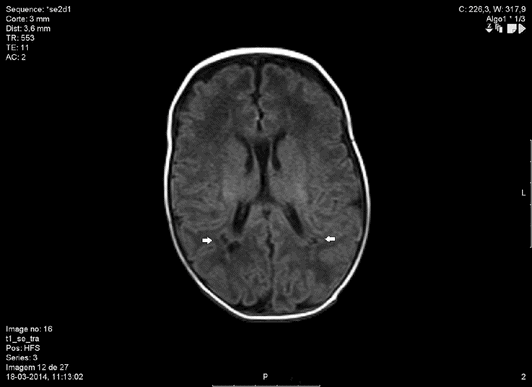
MRI showing cystic periventricular leukomalacia (arrows).
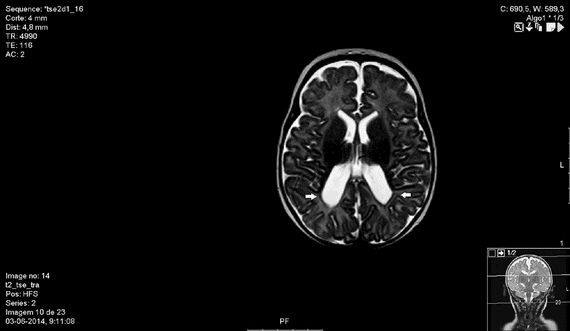
MRI showing subcortical white matter injury, enlargement and irregularity of the ventricular walls (arrows), suggesting periventricular leukomalacia.
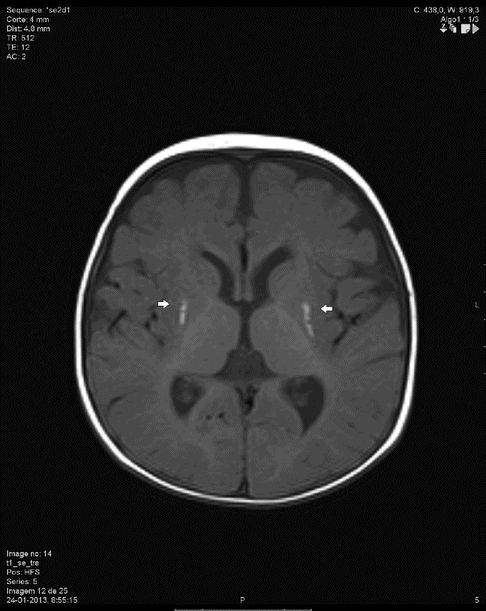
MRI showing lesions of the basal ganglia – lenticular nucleous (arrows).
Individuals with CP, particularly in its most severe forms, frequently have associated neurological abnormalities, namely intellectual disabilities in ∼50% of cases, epilepsy in 25–45%, speech and language disorders in 40%, visual deficits in 40% and hearing impairments in 10–20% [7]. Neurodevelopmental disorders are also common, and features of the autism spectrum disorder may appear in up to 7% of children. Other organs and systems that may be affected are the somatosensory (stereognosis and proprioception impairments), genitourinary (dysfunctional voiding and a high rate of urinary infections), gastrointestinal (dysphagia, oesophageal and bowel dismotility), endocrine (growth failure and osteopenia) and musculoskeletal (subluxation, progressive dysplasia of the hip, foot deformities and scoliosis). Chronic pulmonary disease is a leading cause of death, due to recurrent pneumonia caused by gastro-oesophageal reflux, palatopharyngeal in-coordination and aspiration of gastric content, as well as to restrictive lung disease related to scoliosis.
In an attempt to establish an accurate, reliable and standardized classification of the severity of CP, Palisano et al. developed the Gross Motor Function Classification System, based on the child’s gross motor and functional disabilities established by the International Classification of Impairments, Disabilities and Handicaps [8]. It comprises five levels of dysfunction, according to four different age groups. This classification presents a high inter-rater reliability (particularly after the age of two years) and has been successfully implemented in a variety of settings, including clinical care, research and healthcare administration [9]. Several complementary tools have also been developed to assess fine motor function, such as the ABILHAND-Kids, the Bimanual Fine Motor Function classification and the Manual Ability Classification System [2].
Diagnosis of Cerebral Palsy
Although CP is a static, non-progressive disorder, its clinical expression may evolve as the nervous system matures [10]. Due to the transitory nature of some abnormalities and the low inter-observer agreement associated with their evaluation, a definitive diagnosis usually requires repeated observations and should be deferred until later infancy, particularly in preterm infants. Isolated abnormalities may resolve progressively after nine months of age, spasticity may not be apparent until six months of age and dyskinetic patterns are typically identified only after 18 months. Despite some disagreement as to the age required for an accurate diagnosis, 36 months usually allows a reliable assessment of motor capacity, in contrast to the false-positive diagnoses that may occur before 18 months of age [11].
CP is a diagnosis of exclusion, so atypical neurological signs should raise the possibility of alternative conditions, including neurodegenerative diseases, inborn errors of metabolism, congenital brain and spinal cord malformations, traumatic lesions, neuromuscular/movement disorders and neoplasms.
Neuroimaging methods do not establish a definite diagnosis of CP, but have been used for early prediction of the disease and for excluding other entities (see below). Neurological, neuromotor and neurophysiological tests have also been used with the same purpose. Among the neuromotor assessment tools assisting in the early diagnosis of CP are the General Movement Assessment, with an estimated sensitivity of 98% (95% CI 74–100%) and specificity of 91% (95% CI 83–93%), the standardized Lacey Assessment of Preterm Infants and the Hammersmith Infant Neurologic Examination.
Pathogenesis and Risk Factors
Cerebral palsy has been linked to a wide range of antenatal, intrapartum and postnatal factors that most likely act along the common pathogenic pathways of hypoxia/acidosis, inflammation and neurodevelopmental abnormalities.
The most commonly reported risk factors for CP are prematurity, low birth weight, intrapartum hypoxia/acidosis, chorioamnionitis, central nervous system malformations, intrapartum fever, multiple gestations, coagulation disorders, ischaemic stroke, maternal thyroid disease, obesity and placental pathology [3,12–14].
Prematurity arises as the single most important risk factor for CP, with a magnitude of risk that is inversely correlated with gestational age and birth weight. The prevalence of CP varies from 90 cases per 1000 neonatal survivors of less than 1000 g, to 1.5 cases per 1000 neonatal survivors weighing more than 2500 g [1]. A recent systematic review of 49 studies reported the highest prevalence in neonates weighing 1000–1499 g (59.2 per 1000 live births; 95% CI 53.06–66.01) and in those born before 28 weeks’ gestation (111.8 per 1000 live births; 95% CI 69.53–179.78) [15]. A strong association was also found between multiple gestation and CP, and although birth weight and gestational age are obvious confounders, an independent four-fold increase in risk was reported in a large study evaluating different populations [16].
A substantial body of evidence indicates that a hostile inflammatory environment is another pathogenic pathway leading to CP. Funisitis, increased cytokine levels in amniotic fluid and in fetal blood have all been associated with white matter injury [17]. Maternal bacteriuria (OR 4.7, 95% CI 1.5–15.2) and antibiotic treatment during pregnancy (OR 6.3, 95% CI 3.0–15.2) are independently associated with an increased risk of spastic hemiplegia, and less pronouncedly with diplegia and tetraplegia [18]. A recent systematic review reported a significant association between clinical and histological chorioamnionitis and CP, with pooled odds ratios of 2.42 (95% CI 1.52–3.84) and 1.83 (95% CI 1.17–2.89) respectively. However, there is no robust evidence to suggest that identification and treatment of these infections will alter the course of the disease [19].
Recent data also support the contribution of multiple genetic factors to the aetiology of CP. The prevalence of congenital anomalies in individuals with CP (11–32%) is significantly higher than in the general population (2–3%). Several single-gene Mendelian disorders, inherited as autosomal dominant, autosomal recessive or X-linked, often present with clinical features similar to CP. Many of these gene disorders appear to be responsible for other neurodevelopmental disorders such as autism, attention deficit disorder, intellectual impairment and epilepsy. Despite the growing body of evidence for the genomic causes of CP, in many countries comprehensive genetic testing is still not offered as part of the diagnostic investigation in affected individuals.
Intrapartum hypoxia/acidosis is perhaps the most studied risk factor for CP, with the clear limitation that an agreed set of criteria to define the former entity has not existed. Nevertheless, even with previously existing markers, several large population-based studies, conducted over different time frames and in different populations, have led to the widely accepted conclusion that intrapartum hypoxia/acidosis accounts for only 10–20% of CP cases [1,20–22]. Many intrapartum events have been associated with CP, including instrumental vaginal delivery, caesarean section (CS) and breech delivery [14]. Other related factors are meconium-stained amniotic fluid, meconium aspiration, severe placental vascular lesions, placental abruption and cord prolapse. On the other hand, a recent meta-analysis including a total of 3810 participants found no association between the use of elective caesarean delivery and CP. An increased risk of CP (OR 2.17, 95% CI 1.58–2.98) was found in emergency CSs, although the indication leading to surgery is a likely confounder [23]. Low five minute Apgar scores, seizures, respiratory distress syndrome, hypoglycaemia, jaundice, hyperbilirubinaemia, neonatal sepsis and meningitis have all been reported as neonatal risk factors for CP [13,14]. Perinatal stroke has been the focus of many studies and is correlated with other antenatal and perinatal risk factors for CP, such as intrauterine growth restriction, pre-eclampsia, infection and thrombophilia, although the evidence regarding the latter remains controversial [13].
Consaguinity, rhesus isoimmunization, iodine deficiency and maternal infections due to rubella and cytomegalovirus have also been associated with neurodevelopment abnormalities and CP [1].
Cerebral Palsy Arising from Events in Labour
The multidisciplinary International Cerebral Palsy Task Force published a consensus statement in 1999 defining the criteria necessary to establish a link between intrapartum hypoxia/acidosis and CP [24]. The subject was also analysed in 2003 by the American College of Obstetricians and Gynecologists (ACOG) together with the American Academy of Pediatricians (AAP). More recently, FIGO published its revised consensus on intrapartum fetal monitoring where the causal relationship between hypoxia/acidosis and CP is also evaluated [22,24,25]. There is a general agreement that a number of findings are necessary to implicate intrapartum hypoxia/acidosis as the possible cause of CP in term infants (Table 31.1).
1. Metabolic acidosis in umbilical artery blood or in newborn circulation during the first minutes of life (pH <7.00 and base deficit ≥12 mmol/l or lactate ≥10 mmol/l) 2. Low Apgar scores at one and five minutes 3. Early onset hypoxic-ischaemic encephalopathy grade 2 or 3 4. Early imaging studies suggestive of an acute and non-focal cerebral anomaly 5. Spastic quadriplegic or dyskinetic cerebral palsy 6. Exclusion of other identifiable aetiologies such as birth trauma, coagulation disorders, infection and genetic disorders |
The application of the ACOG/AAP 2003 criteria was retrospectively evaluated by Phelan and coworkers in 39 neonates with permanent neurologic impairment. Three or more of the essential criteria were met in 94% of cases. Umbilical artery pH below 7.00 was found in 97% of cases, base deficit equal or above 12 mmol/l in 100%, moderate or severe encephalopathy in 97%, spastic quadriplegic or dyskinetic cerebral palsy or death attributable to brain injury in 94%. None of these neonates had any other identifiable aetiologies for their CP [26].
No currently available clinical procedure, including continuous cardiotocographic (CTG) monitoring during labour or increased CS rates, has been shown to reduce the risk of CP in term infants. Continuous intrapartum CTG was compared with intermittent auscultation in several randomized trials carried out before 1993 [27]. While it was associated with a 50% reduction in neonatal seizures, no effect was evident in the incidence of CP or perinatal mortality, but the studies were clearly underpowered to show a difference in these rare outcomes. Moreover, the technology has evolved since that time, so the existing evidence comparing continuous CTG with intermittent auscultation regarding the incidence of CP is scientifically inconclusive.
Hypoxic-ischaemic Encephalopathy
Although there is no universally accepted definition, neonatal encephalopathy is a clinical syndrome of near-term neonates that manifests with an abnormal level of consciousness, or seizures, difficulty with initiating and maintaining breathing and a depression of tone and reflexes [28]. It occurs in about 3 per 1000 term live births [29] and is considered the strongest clinical predictor for the occurrence of CP.
Hypoxic-ischaemic encephalopathy (HIE) is a form of neonatal encephalopathy that is caused by intrapartum hypoxia/acidosis, and has an estimated incidence of 1.5 per 1000 live births [29]. Since there are multiple non-hypoxic causes for neonatal encephalopathy, in order to establish the diagnosis of HIE it is necessary to document the occurrence of metabolic acidosis in umbilical cord blood or in the newborn circulation during the first minutes of life [30], together with low Apgar scores and early imaging evidence of cerebral oedema. It is not infrequent for there to be some uncertainty as to the exact cause of neonatal encephalopathy.
According to the Sarnat & Sarnat classification, three grades of HIE are distinguished: grade 1 is characterized by hyperalertness, irritability, jitteriness and a normal electroencephalogram; grade 2 is characterized by obtundation, hypotonia, strong distal flexion, occasional multifocal seizures and has a 20–30% risk of death or major neurological sequelae such as CP; grade 3 is associated with coma, and in the majority of cases there is neonatal death or long-term neurological sequelae such as CP [31]. In a recent systematic review, 14.5% of HIE cases were reported to develop CP [21]. The co-existence of multisystem organ failure, including renal, hepatic, haematologic, cardiac, metabolic and gastrointestinal functions, is frequent in HIE, but the severity of neurological injury does not necessarily correlate with the involvement of other systems.
Similarly to CP, an estimated 70% of cases of neonatal encephalopathy is thought to be consequent to events arising before the onset of labour [22], and less than 10% due to postnatal complications such as severe respiratory distress, sepsis and shock [32]. Most of the remaining cases are due to intrapartum hypoxia/acidosis, but their incidence varies according to the healthcare setting. Hypoxia/acidosis can also occur during pregnancy and in the postnatal period and cause neonatal encephalopathy, but these situations will not display metabolic acidosis at birth. The occurrence of clinical ‘sentinel events’ may help to differentiate between a prenatal and an intrapartum event, namely when there is documentation of prolonged maternal hypotension, maternal cardiovascular collapse, ruptured vasa praevia, massive feto-maternal haemorrhage, uterine rupture, major placental abruption, umbilical cord prolapse, shoulder dystocia and retention of the after-coming head. A CTG tracing acquired in the beginning of labour may also help to determine the timing of damage. Reduced variability and absence of accelerations lasting 50 min or more suggests the possibility of a previously damaged fetus, while a normal tracing that later on becomes pathological is more likely to be associated with an intrapartum event [25].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


