Grade
Modified Tardieu scale
Modified Ashworth scale
0
No resistance to passive ROM
No increase muscle to passive ROM
1
Slight resistance with no clear catch
Slight increase in tone, catch, and release or minimal resistance with ROM
2
Clear catch at precise angle, halting passive ROM, followed by release
Marked increase in muscle tone, catch in middle range and resistance through remained of ROM, still easily moved
3
Fatigable clonus (<10s with resistance) occurring at precise angle
Considerable increase in muscle tone, passive movement is difficult
4
Infatiguable clonus (>10s)
Rigid in flexion or extension
Dystonias are the more difficult type of tone for the orthopedic surgeon to evaluate and treat, as it does not allow for predictable outcomes to surgical interventions and in many cases is a contraindication. However, dystonia often accompanies spasticity to varying degrees. Cocontraction in dystonia is not always apparent during initial exam, particularly when spasticity predominates. The practitioner is cautioned that interventions may unmask dystonia with opposite results of the intended procedure.
Multiple rating scales exist for dystonia and have been tested for reliability and validity in CP. None have been found superior. These include the Barry-Albright Dystonia Scale, Burke-Fahn-Marsden Scale, Burke-Fahn-Marsden Movement Scale, and the Unified Dystonia Rating Scale (Monbaliu et al. 2010). These scales are not commonly used by orthopedic surgeons in clinical practice.
Strength is assessed using manual muscle testing and compared bilaterally. Typical scales are 0–5, 0-no contraction and 5-full range of motion against maximum resistance. Dynamometry may also be used (Crompton et al. 2007).
Selectivity of muscle or muscle groups is graded 0–2, where 0 is patterned movement, 1 is partially isolated movement, and 2 is completely isolated movement.
Reflexes tested include deep tendon reflexes of the biceps, brachioradialis, and triceps. Hoffman’s sign or increased finger flexor reflexes may be present and are consistent with an upper motor neuron lesion.
Associated injuries, such as sensory and perception disturbances, are evaluated using stereognosis testing. Stereognosis testing assesses the perception/sensation impairment of the extremity and is a valuable component to functional testing and educational for patient and caregivers. Gross and fine objects are placed into the patient’s hand, and patient is asked to identify the object (Van Heest et al. 1993; Carlson and Brooks 2009; Yekutiel et al. 1994; Goldner 1966; Gordon and Duf 1999).
Functional tests are many. Several have been validated in children as well as in the CP population. These include: Manual Ability Classification System (MACS) (Gong et al. 2010), the Shriner’s Hospital for Children Upper Extremity Evaluation (SHUEE), the Jebsen-Taylor Hand Function Test, House, and the Assisting Hand Assessment (AHA) (Davids et al. 2006). The House and AHA are specific to hemiplegia. Hemiplegic hand function can be documented in a busy upper extremity clinic; using the House examination, see Table 2 (House et al. 1981; Morris et al. 2006; Van Heest 2003b; Bolanos et al. 1989). The AHA requires special training and more time to administer, but also evaluates the extremity in usual situations of play and daily bimanual activities.
Class | Designation | Activity level |
|---|---|---|
0 | Does not use | Does not use |
1 | Poor passive assist | Uses as a stabilizing weight |
2 | Fair passive assist | Holds object placed into hand |
3 | Good passive assist | Holds and stabilizes object for use by other hand |
4 | Poor active assist | Actively grasps object and holds weakly |
5 | Fair active assist | Actively grasps object and stabilizes well |
6 | Good active assist | Actively grasps object and manipulates against other hand |
7 | Spontaneous partial use | Carries out bimanual activities easily and occasional spontaneous use |
8 | Spontaneous use complete | Uses hand independently |
The Manual Ability Classification System (MACS) was developed to describe how children with cerebral palsy use their hands to handle objects in daily activities and reflects the child’s typical performance. It has been tested and validated for use in children from 4 to 18 years of age. It ascribes a level from I to V of manual ability; see Table 3 (Morris et al. 2006; Eliasson et al. 2006; Öhrvall et al. 2014).
Table 3
Manual Ability Classification System (MACS) levels
Level 1 | Handles objects easily and successfully |
Level 2 | Handles most objects but with somewhat reduced quality and/or speed of achievement |
Level 3 | Handles objects with difficulty, needs help to prepare and/or modify activities |
Level IV | Handles a limited selection easily managed objects in adapted situations |
Level V | Does not handle objects and has severely limited ability to perform even simple actions |
Outcome measures include the Canadian Occupational Performance Measure (COPM), which is validated for use in CP as well as pediatrics, and the Pediatric Outcomes Data Collections Instrument (PODCI).
Electromyography (EMG)
Dynamic electromyography (EMG) is helpful in determining the phasic nature of muscle spasticity when entertaining the possibility of a muscle transfer or release (Keenan et al. 1990). Though some studies have shown that muscles can change phase after transfer, it is our practice to attempt to use in phase muscles for transfers when possible. Transferring in phase muscle facilitates synergistic movements and eases rehabilitation (Mowery et al. 1985). EMG is also useful in determining if cocontraction is present during specific tasks or movements, particularly in patients with dystonia (Malfait and Sanger 2007). Traditional EMG may be used to determine underlying or resting muscle activity. EMG can be performed using surface electrodes or needle electrodes (Hoffer 1979, 1993a, b).
Cerebral Palsy of the Upper Extremity Treatment Options
It cannot be overemphasized that the management of patients with CP requires a multidisciplinary team and must involve the patient and family/caregivers in developing the child’s individualized treatment plan. Although they will be discussed separately, nonoperative and operative treatment is additive, and successful outcomes are best achieved with integrated care approaches.
Nonoperative Management of Upper Extremity Cerebral Palsy
Therapy
The importance of therapy in CP has been proven, and patient outcomes regardless of the operative or nonoperative intervention rely on adherence to therapy protocols. Caregivers’ and patients’ involvement in home exercise programs is critical (Sakzewski et al. 2011, 2014; Scholtes et al. 2010). Therapy is the mainstay of nonoperative management, but is equally if not more important when undertaken perioperatively (Speth et al. 2005).
Therapy modalities include strengthening, stretching, functional tasking, ADLs, sensory education, and electrical stimulation. Therapists use varying techniques including splinting, casting, ultrasound, and electrical stimulation (Sakzewski et al. 2014).
Splinting and Casting
Splinting for functional assistance, such as wrist stabilization in neutral position for hand function, is common using both custom and prefabricated orthoses. Splinting to prevent contractures is typically used at night and is directed to the most common deformities, such as elbow flexion, wrist flexion, finger flexion, and thumb adduction (Louwers et al. 2011; Imms 2011).
Serial casting is used for contractures. This may or may not be accompanied by concomitant botulinum toxin injections or other chemodenervation. Serial casting is performed by the physician or qualified therapist, and casts are changed weekly for 3–6 weeks. Casting is followed by therapy and night splinting to maintain the position attained.
Constraint-Induced Therapy
Constraint-induced therapies (CIT) stem from use in stroke patients to reeducate and organize the injured and healing brain. There is some controversy in upper extremity CP management of the use of CIT. Several studies have documented improved extremity position and function in the CP population (Crocker et al. 1997). Protocols for CIT vary widely, but involve some form of constraint of the better functioning upper extremity and intense therapy and functional training of the involved extremity. This may or may not be followed by bimanual training/therapies (Sakzewski et al. 2011, 2014; Eliasson et al 2014; Tona and Schneck 1993; Pierce et al. 2002).
Electrical stimulation (E-stim) paired with splinting or bracing and therapy protocols have shown some promise in patients with spasticity and without static contractures (Ozer et al. 2006; Carmick 1993). This involves superficial electrodes applied to the weaker extensor muscles, such as the extensor digitorum communis and the triceps and placing the flexors on mild stretch. Protocols vary in frequency and duration, but are typically performed at home daily for 4–12 weeks and then placed on a maintenance schedule. E-stim can also be a useful adjunct to chemodenervation, using E-stim to the triceps following botulinum toxin injections to the biceps and brachialis for elbow flexor spasticity (Scheker et al. 2003; Ozer et al. 2006; Wright and Granat 2000a, b).
Cerebral palsy of the upper extremity | |
|---|---|
Nonoperative management therapy | |
Indications | Contraindications |
Muscle balance across joints, joint posture | Pain, recalcitrant contractures |
Activities of daily living and independence | Poor voluntary control of the extremity |
Pre- and postoperative care | Noncompliance, limited access, socioeconomic issues |
Chemodenervation
Chemodenervation is commonly used in the treatment of focal spasticity. Phenol, ethanol, and botulinum toxins are the most commonly used. Injections are intramuscular and may be guided by ultrasound (US), electromyography (EMG), electrostimulation, or surface anatomy (Elovic et al. 2009). Botulinum toxin injections have been studied both in the upper and lower extremities in the treatment of muscle spasticity and abnormal muscle tone. It has been shown to improve position and range of motion and sometimes function. The effect on the muscle is temporary, and the temporal effect of the improvement has not been consistently quantified, but ranges from 3 to 12 months duration (Wall et al. 1993; Wallen et al. 2007; Barber et al. 2013). The use of chemodenervation in dystonia is encouraging, where surgical interventions are of limited usefulness. The botulinum toxin injections may help in delaying surgical releases and/or predict the results of a tendon or muscle release (Autti-Ramo et al. 2000; Van Heest 1997; Speth et al. 2005; Barrett 2011; Sakzewski 2010; Koman et al. 1990, 1993, 2003, 2010; Friedman et al. 2000; Corry et al. 1997; Anakwenze et al. 2013). Botulinum toxin injections may be used as an adjunct to surgical procedures, to aid in postoperative muscle spasticity and pain following muscle/tendon lengthenings or transfers (Yang et al. 2003).
Cerebral palsy of the upper extremity | |
|---|---|
Nonoperative management chemodenervation | |
Indications | Contraindications |
Focal or multi-segmental spasticity | Widespread tone → high doses |
Dynamic contractures | Severe static contractures |
Dystonia | History of allergic reaction |
Pharmacologic Management
Systemic management of the movement disorders in CP should also take into account the many associated disorders, such as epilepsy and behavior disorders. The medications may interact or overlap. A physiatrist, neurologist, or developmental pediatrician typically oversees and manages these therapies. Medications include baclofen (oral or intrathecal), benzodiazepines such as diazepam and clonazepam, dantrolene sodium, and tizanidine (Watanabe 2009; Francisco et al. 2009; Bonouvrié et al. 2013; Nogen 1976).
Many of these medications cause sedation, weakness, and central depression. Therefore, their use is often limited in the higher functioning patients, who do not tolerate or appreciate these side effects. The medications are more often used in the more involved and refractory patients.
Cerebral palsy of the upper extremity | |
|---|---|
Nonoperative medical systemic management | |
Indications | Contraindications |
Nonfocal and severe spasticity or dyskinetic CP | Focal spasticity |
Associated disorders, i.e., seizure disorder | Minimal cognitive impairment |
High surgical risks | No associated disorders |
Cerebral palsy |
|---|
Physical/occupational therapy recommendations |
Maintain supple range of motion |
Prevent contracture |
Maximize independence |
Bimanual tasks |
Adjunct to all other nonoperative and operative interventions |
Nonoperative Management Outcomes
Resistance exercise strengthening in the lower extremities of children with CP was compared to traditional therapies in a randomized trial. The experimental group had muscle strength increases up to 14 %, but did not demonstrate improved mobility (Scholtes et al. 2010). The effects of wearing a thumb and wrist brace on bimanual activities in children with hemiplegia noted significant improvement in spontaneous use and bimanual activities (Louwers et al. 2011). Constraint-induced therapy (CIT) compared with bimanual training (BIM) did not demonstrate significant differences, but did show that CIT yielded greater unimanual improvement and BIM yielded improved bimanual function; thus, perhaps both therapies play a role in functional improvements (Sakzewski et al. 2011; Scheker and Ozer 2003; Law et al. 1991).
Short-term outcomes in a randomized trial have shown botulinum toxin A added to an intensive therapy regimen has improved functional outcomes compared to intense therapy alone (Speth et al. 2005). E-stim and dynamic bracing combined was compared with either therapy alone in a randomized trial. The combined therapy yielded functional and postural improvement over either therapy alone; however, after 2 months results faded. The authors concluded that the therapy had to remain ongoing to maintain benefits (Ozer et al. 2006; Scheker et al. 1999; Scheker and Ozer 2003; Hines et al. 1993). Recently, a multicenter prospective study compared therapy alone, botulinum toxin, and surgery in children with hemiplegia. The children in the surgical group had greater improvement in upper limb positioning compared to the other groups (Van Heest et al. 2013).
Operative Treatment of Cerebral Palsy
Indications/Contraindications
Extensive interviews of the child, family, therapists, and other caring physicians are requisite to avoid complications and navigate surgical decision making in children with cerebral palsy. Multiple clinic visits as well as observing the patient during activities (functional testing, videotaping) and/or ambulation are necessary to capture such a dynamic clinical exam (Van Heest et al. 1999). Use of preoperative botulinum toxin injection trials into spastic muscles and EMG may help predict surgical outcomes and set expectations for surgical results. Ultimately, surgical indications and timing must be individualized to each child and caregiver team. The importance of the patient’s age and growth remaining cannot be overemphasized when formulating the treatment plan. Longitudinal growth will increase the likelihood of contractures and recurrence of contractures postoperatively (Swanson 1960, 1964, 1968, 1982; Mital and Sakellarides 1981).
In the carefully selected patient, surgery will provide discrete benefits over nonoperative treatments for hygiene, form, and functional improvement in the upper extremity in children with cerebral palsy (Waters and Van Heest 1998; Stelling and Meyer 1959; Skold et al. 2007; Roth et al. 1993; Pontén et al. 2011; Johnstone et al. 2003; Dahlin et al. 1998). Ongoing large multicenter clinical studies and centuries of published literature have described a variety of surgical procedures for improvement in the position. Overwhelmingly, performing multiple procedures at the same time to “rebalance” the entire extremity is advocated (Smitherman et al. 2011; Samilson 1966; Samilson and Green 1972; Nylander et al. 1999; Hoffer 1989, 1986; Gelberman 1991; Eliasson et al. 1998). Below, listed by anatomic location, are the indications, contraindications, techniques, pitfalls, pearls, and postoperative protocols for the most commonly utilized procedures in reconstruction of the upper extremity for children with cerebral palsy (Zancolli and Zancolli 1981; van Munster et al. 2007, 2009; Szabo and Gelberman 1985; Samilson and Morris 1964; Pollock 1962; Goldner 1955, 1961, 1974, 1979, 1983, 1987, 1988).
Dyskinetic movement disorders, continuous spastic muscle tone, poor distal sensibility and stereognosis, and limited voluntary control of the upper extremity are relative contraindications to soft tissue procedures. These findings guide surgical decision making toward nonoperative treatment and/or fusions and salvage procedures (Skoff and Woodbury 1985; Manske and Strecker 1996; Lynn et al. 2009; Lomita et al. 2010; Cooper 1952).
Surgical Procedures
Elbow
Preoperative Planning
The elbow positions the hand in space for function. If the hand cannot rest on the tabletop or reach out for objects secondary to elbow flexion contracture, elbow contracture release may increase upper extremity function (Morrey et al. 1981). Children with cerebral palsy most often demonstrate spasticity of their elbow flexor muscles (biceps, brachialis, and brachioradialis) as well as weakness in their elbow extensors (triceps). This muscle imbalance can lead to flexor muscle and elbow joint contractures. With the long bone growth of the humerus, elbow contractures can worsen during growth spurts and stabilize with skeletal maturity. Finally, the elbow crease can be a source of intertriginous infection and skin breakdown. Elbow antecubital crease hygiene is also an indication for surgery (Sherk 1977; Dy et al. 2013)
Preoperatively, the surgeon should determine the structures responsible for the elbow contracture. Dynamic contractures, which correct with passive stretch, can be improved with muscle or tendon lengthening. Fixed flexion contractures of the elbow may require additional fasciotomies, capsulotomies, myotomies, and skin releases (Pletcher et al. 1976; Hotchkis 2011).
Upper extremity reconstruction in CP |
|---|
Preoperative planning |
OR table: regular |
Position/positioning aids: supine/hand table |
Fluoroscopy: required only for arthrodesis or osteotomy |
Equipment: hand instrument tray |
Tourniquet: sterile tourniquet upper arm |
Surgical Approach
Elbow flexion contractures are approached through the antecubital fossae. The internervous plane of dissection is between the biceps (musculocutaneous n.) and brachioradialis (radial n.). Across the antecubital fossae, the biceps tendon lies lateral to the brachial artery and the median nerve. In severe elbow flexion contractures, bowstringing of the neurovascular structures can change the planes of dissection and ultimately limit the elbow release. When combined with forearm, wrist, or hand surgery, the elbow contracture is addressed first to allow for improved positioning of the forearm for the more distal procedures (Mital 1979).
Technique: Elbow Flexion Contracture Release
The patient is placed supine on the regular OR table with the entire upper extremity prepped, draped, and outstretched on the hand table. If the child has severe shoulder adduction contracture, position the hand table where the elbow can be accessed most comfortably. Tourniquet may be used at the surgeon’s discretion. When used, a sterile tourniquet is placed, the arm is exsanguinated with an esmarch wrap with the arm in extension, and the tourniquet is elevated. Skin incisions can be a transverse antecubital incision or a curvilinear incision or “lazy S,” moving lateral proximal (to see the radial nerve) and medial distal. In severe contractures, a well-designed single or multiple Z-plasty may be necessary to release antecubital skin pterygium resulting from chronic fixed contractures. All incision options are centered over the palpable lacertus fibrosus and biceps tendon and extending wide enough to visualize and protect the lateral and medial neurovascular structures.
Once the skin is incised, antecubital veins are mobilized and protected. The terminal branch of the musculocutaneous nerve, the lateral antebrachial cutaneous nerve, tracts just lateral to the biceps tendon and is identified, protected, and retracted. The lacertus fibrosus is identified and divided, leaving the entire biceps tendon intact. If the patient has a mild dynamic contracture, fractional lengthening of the biceps may be performed by dividing the tendinous portion transversely at the musculotendinous junction, keeping the muscle in continuity.
If the child has muscle contracture , joint involvement, or moderate dynamic elbow spasticity, then the biceps tendon is Z-lengthened. The entire length of the biceps tendon is dissected and visualized. A natural raphe exists splitting the tendon. Open the raphe longitudinally, and cut the medial half of the tendon proximally and the lateral half distally. In a severe elbow contracture, predict the expected lengthening needs for optimized elbow position. In dynamic contractures, limit lengthening to 2 cm to avoid extreme weakening.
Through the arms of the biceps Z-plasty or retracting the biceps if it is not lengthened, identify the median nerve and brachial artery and protect them medially. The brachialis muscle is then exposed. Dissect between the brachialis and brachioradialis muscle to visualize the radial nerve. A fractional lengthening of the brachialis muscle is then performed based on severity of the elbow involvement. The thickest part of the brachialis tendon is lateral, right next to the radial nerve. The tendon is divided transversely from lateral to medial at the musculocutaneous junction with two parallel incisions approximately one centimeter apart (Manske et al. 2001).
Once the biceps and brachialis are lengthened, assess the fascia over from the pronator mass and extensor masses cross the elbow. In older patients, this will thicken and be an additional obstacle to elbow extension. The brachioradialis is then exposed and evaluated. If you plan to use it as a donor muscle for distal transfer, lengthening is not recommended. If no transfer is planned, fractional lengthening of the brachialis as it crosses the elbow will allow additional elbow extension.
Complete brachialis myotomy is indicated only in severe contractures for hygiene issues. Performing the myotomy with a coated needle-tip bovie may decrease postoperative bleeding. If a substantial contracture is still present, capsulotomy is performed. The anterior capsule can be released from the anterior humeral periosteum with an elevator while applying gentle elbow extension force to facilitate capsular release. Ultimately, the neurovascular bundles medially and laterally will bowstring and may limit the degrees of elbow extension obtainable. In these cases, deflate the tourniquet prior to elbow casting, and place the elbow in a position that ensures blood flow and capillary refill in the hand. Translate the ends of the biceps tendon, and repair them side by side, or weave them together into the optimized lengthened position with 2-0 nonabsorbable suture.
The skin is then closed with absorbable deep and subcutaneous suture. Consider placing a drain if a myotomy was performed to prevent hematoma. A long arm cast or splint is applied. When a mild dynamic elbow contracture release is performed simultaneously with distal forearm, wrist, and/or hand procedures, the distal short arm cast will act like a weight, and patient can begin gentle active flexion of the well-repaired biceps within a few days. If an extensive skin, muscle, and/or joint contracture of the elbow has been performed, use a cast or splint to maintain elbow extension for a few weeks. If a forearm procedure is performed, the elbow must be immobilized with the forearm in the desired position to prevent recurrence of forearm contractures. See Fig. 1.
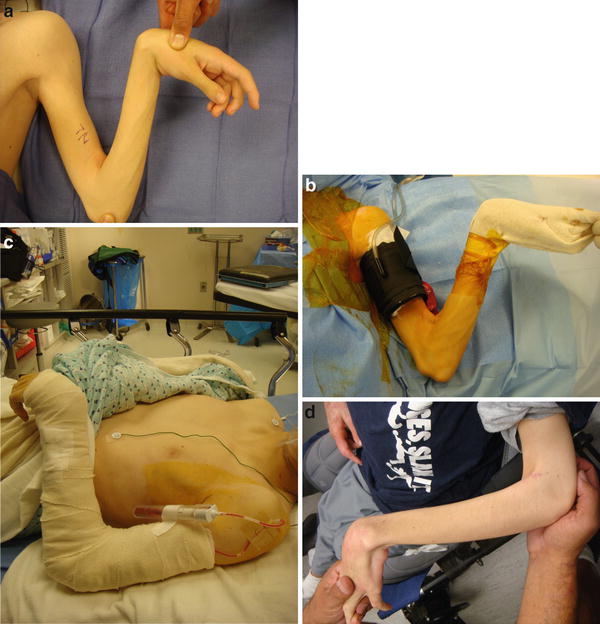

Fig. 1
(a) Preoperative photograph of elbow flexion contracture. (b) Intraoperative positioning. (c) Immediate postoperative photograph. (d) Postoperative follow-up photograph
Elbow contracture release in CP |
|---|
Surgical steps |
Transverse, curvilinear, or Z-plasty skin incision |
Identify and protect: antecubital veins, lateral antebrachial cutaneous nerve, median nerve, brachial artery, radial nerve |
Transect the lacertus fibrosus |
Fractional or Z-lengthen the biceps tendon |
Fractional lengthening or myotomy of the brachialis muscle |
Expose and release the anterior capsule if needed |
Release the fascia over the brachioradialis and flexor-pronator mass as they cross the elbow |
Repair the biceps in a lengthened position if a Z-plasty completed |
Close the skin over a drain if myotomy of brachialis was performed |
Elbow flexion contracture release in CP |
|---|
Postoperative protocol |
Dynamic contractures do not require casting or splinting and can begin active range of motion immediately |
Plaster anterior splint or cast of elbow in maximized extension for severe fixed contractures with neurovascular checks |
If immobilized, keep in place x 4–6 weeks |
May begin active and passive range of motion immediately following splint or cast removal |
Occupational or physical therapy protocols and home exercise program to maintain results. Night splinting to prevent recurrence |
Activities of daily living training and return to sports at 3 months |
Surgical Pitfalls and Prevention
Elbow flexion contracture release surgery in cerebral palsy | |
|---|---|
Potential pitfalls and preventions | |
Potential pitfall | Pearls for prevention |
Overlengthening the biceps tendon | In dynamic contractures limit lengthening to <2 cm |
Long side-by-side repair or weave | |
Postoperative hematoma | Use of a coated needle-tip bovie for myotomy |
Careful identification of neurovascular structures and protection of antecubital veins | |
Neurovascular compromise from bowstring and traction on antecubital structures | Limit retractors on structures to avoid injury |
Respect limit of achievable elbow extension with neurovascular checks postoperatively and splint position adjustments | |
Musculocutaneous Neurectomy
Musculocutaneous neurectomy, or selective peripheral neurotomy (SPN) for complete denervation of the biceps and brachialis muscles (Maarrawi et al. 2006; Sindou et al. 2007), is a salvage procedure for elbow spastic deformity. Active elbow flexion is possible through the remaining brachioradialis. It is contraindicated in patients with functional elbows. Neurectomy addresses only muscular spasticity and is relatively contraindicated when fixed contracture exists or the elbow contracture must be addressed in conjunction (Purohit et al. 1998). Sensory deficit in the lateral arm will result from denervation of the lateral antebrachial cutaneous nerve. Preoperative lidocaine block of the musculocutaneous nerve or EMG can help predict outcomes.
Technique: The patient is placed supine on the operating table with the arm extended over a hand board. The upper extremity and chest are prepped with split drapes to midline to allow access to the sterile axilla. A curvilinear incision is made along the crease of the axilla. The fascia over the brachial plexus cords and nerve branches is opened, and the musculocutaneous nerve is identified emanating from the lateral cord of the brachial plexus next to the biceps tendon. The nerve is followed distally toward its innervation to the biceps muscle. The nerve is then transected, and a one centimeter segment is removed. The axillary incision is closed primarily. An adhesive waterproof dressing or bulky shoulder wrap that incorporates the axilla is placed. The elbow is kept clean and dry for 10–14 days. No postoperative immobilization is necessary.
Forearm Pronation Contracture
Preoperative Planning
The modern day workspace is predominately tabletop with the forearm in pronation. Activities of daily living such as hygiene, dressing, and eating as well as most sports and instrument play require forearm rotation into varying degrees of supination. Spasticity of the flexor-pronator mass and pronator quadratus muscles as well as weakness of the forearm supinators (biceps and supinator muscles) positions the forearm in hyperpronation. Operative treatment of forearm dynamic and fixed pronation contractures can increase a child’s independent and bimanual function (Gschwind and Tonkin 1992; Gschwind 2003; Colton et al. 1976).
On clinical examination or videotape functional testing, the patient should be asked to actively supinate. If the patient can actively supinate to a functional position, no surgery is indicated, and continued nonoperative strengthening of the supinators should be pursued. If the child cannot actively supinate but can be passively supinated, then their forearm contracture is dynamic, and pronator teres release or rerouting is indicated (Manske and Strecker 1987). The rerouting should only be performed if the pronator teres is phasic with attempted supination on dynamic EMG. If the pronator teres is continuously active on EMG, release should be performed (Strecker et al. 1988; de Roode et al. 2010). If the child cannot actively or passively supinate, then the contracture is fixed and a pronator teres tenotomy and/or pronator quadratus released, or forearm rotational corrective osteotomies of both the radius and/or ulna may be indicated (Sakellarides et al. 1981; Ezaki and Oishi 2012).
Release of pronator muscles in combination with a supinating wrist extension tendon transfer, such as a flexor carpi ulnaris ( Green transfer) or brachioradialis transfer, may result in overcorrection and decreased function. In addition, if the pronator is released from its origin with a flexor origin slide, then it does not need to be released at its insertion. Careful preoperative evaluation and planning is required to prevent this complication (Bunata 2006). Preoperative botulinum toxin injection trial and/or EMG of the pronator teres and quadratus muscles can be utilized to assess individual muscle contribution and predict surgical outcomes. X-rays of the forearm are utilized to assess radioulnar joint mechanical blocks to rotation or if radial head dislocation, distal radioulnar joint subluxation, or synostosis is suspected (Ozkan et al. 2004; Cheema et al. 2006).
Positioning
The operating room setup, positioning, and preparation are the same as that described for elbow contracture release and most commonly part of multiple procedures performed concurrently with elbow, wrist, and hand reconstruction.
Surgical Approach
Most commonly, the pronator is approached at its insertion on the midshaft of the radius through a direct radial incision. If a concurrent forearm procedure such as a wrist and finger contracture release is planned, surgical incisions may vary if incisions for other planned procedures provide access to the pronator teres and/or quadratus origins and insertions.
Technique: Forearm Pronation Contracture Release
Topographically, the pronator teres can be palpated as it inserts onto the radius as the most superficial and lateral muscle of the flexor-pronator mass. Estimate the incision to access the pronator insertion by tracing the muscle distally; this should bring you to a several centimeter incision on approximately the midshaft of the radius. Dissection through the skin will demonstrate large forearm veins superficially than can be dorsally mobilized or ligated. The lateral antebrachial cutaneous nerve is identified on the surface of the brachioradialis muscle and protected laterally. The brachioradialis is swept dorsally. Care should be taken to prevent retractors on the superficial radial nerve beneath the brachioradialis. The long sweeping insertion the pronator teres is found confluent with the periosteum of the midshaft of the radius. Dissect and retract proximally to isolate the pronator muscle and visualize its musculotendinous junction. Tenotomy of the entire PT tendon is performed under direct visualization. If the rerouting procedure is planned, the PT insertion is lifted with a slip of periosteum from the lateral radius. Once the insertion has been lifted, dissection proximally mobilizes the muscle to its neurovascular pedicle to avoid kinking. Place stay sutures in the end of the tendon. Use a suture passer, a right angle, or curved Satinsky clamp (Pilling Surgical, North Carolina, USA) to pass the tendon through the interosseous membrane and around the dorsal radius. By staying on the radius, the anterior interosseous artery and nerve and radial artery should not be at risk. Use suture anchors, or pass the tendon on a free needle through bone tunnels made with a K-wire or drill, at the level of the PT insertion, and direct the tunnel from dorsal to volar. Position the forearm in full supination. Secure the tendon to the lateral dorsal radius with nonabsorbable suture. Securing the rererouted tendon may best be performed after all other upper extremity procedures are completed so that inadvertent rupture of the repair does not occur (Fig. 2). The forearm should still be able to be passively pronated. The pronator quadratus (PQ) should not be released concurrently with a rerouting, to avoid overcorrection and loss of pronation.
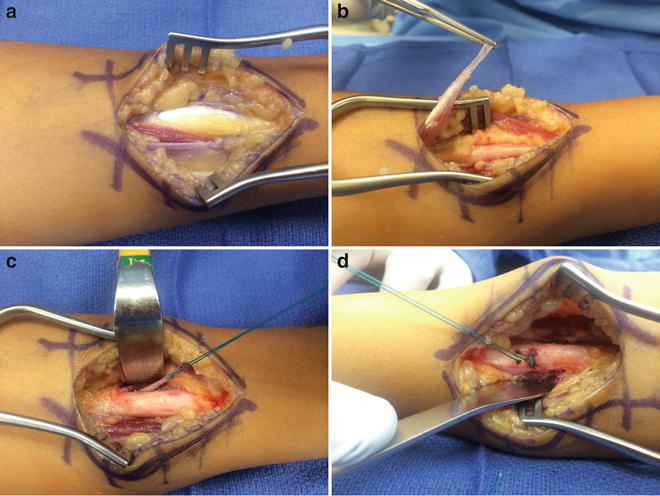

Fig. 2
(a) Exposure of pronator teres (PT) insertion. (b) Elevation of insertion in preparation for rerouting. (c) PT insertion rerouted around the radius. (d) PT sewn through bone tunnels in the radius
If isolated pronator quadratus release is being performed, the PQ release from the radial border is performed through a radial styloid or volar Henry approach.
After either PT/PQ release or PT re-rerouting, perform primary closure, and an above-the-elbow cast or sugar tong splint is placed to keep the forearm in supination for 4–6 weeks. Subsequently, refer to occupational therapy for removable supination splinting for an additional 4 weeks with place, and hold active and passive supination and active pronation exercises. Encourage the child to assume bimanual activities of daily living and play that utilize supination and forearm rotation.
Forearm contracture release in CP |
|---|
Surgical steps |
Curvilinear or oblique direct midshaft radial incision along the PT. Distal radial incision or volar Henry approach to access the PQ. Preoperatively consider all incisions planned as the insertions for the PT and PQ may be accessed through radial styloid or volar incisions made for other concurrent procedures |
Identify and protect the lateral antebrachial cutaneous and superficial radial nerves lying anterior and beneath the brachioradialis muscle, respectively |
Tenotomy or rerouting of the pronator teres muscle at its insertion |
During rerouting procedure, stay along the radius to protect the interosseous ligament and neurovascular structures. Secure the tendon to the dorsal lateral radius through a bone tunnel with the forearm in supination |
If necessary, extend the incision distally. Release the PQ from its most radial attachment while actively supinating the forearm |
Forearm pronation release in CP |
|---|
Postoperative protocol |
Long arm cast in supination or initial sugar tong splint |
4–6 weeks |
Supination active place and hold |
Begin bimanual activities after 6 weeks to encourage supination |
Forearm deformity correction in cerebral palsy | |
|---|---|
Potential pitfalls and preventions | |
Potential pitfall | Pearls for prevention |
Overcorrection | Do not release all pronators |
Recognize that FCU to ECRB is also a supinating transfer | |
Careful pre-op evaluation of all structures to predict outcomes | |
Undercorrection | Identify possible joint mechanical blocks to forearm rotation such as radial head dislocation or synostosis |
Avoid rerouting procedures in patients with fixed contractures | |
Wrist
Preoperative Planning
Wrist position in children with cerebral palsy is intricately involved in finger deformity and overall hand function. Power grip and pinch cannot be achieved through a flexed wrist. Use of communication device or wheelchair can depend on wrist position. Surgical correction of wrist deformity in children with cerebral palsy can open the hand for bimanual activities and increase function and hand strength. However, due to the extrinsic finger flexors crossing the wrist and the almost uniform extensor weakness of the wrist and finger extensors, overcorrection of wrist deformity can actually worsen hand function. Therefore, surgery for the treatment of wrist deformity should only be performed after extensive evaluation of the individual contribution of each wrist and finger flexor and extensor to predict the tenodesis effect after correction of the wrist.
Deformity of the wrist is not only in the flexion/extension plane but also in the radial/ulnar deviation/coronal plane. Surgical correction of lateral plane deviation can be achieved by balancing the insertion location of tendons. Overtime, bony deformity, degenerative changes, and radiocarpal subluxation may occur. Joint resection and salvage procedures can decrease pain associated with deformity and arthritis. In addition, hyperflexion causes difficulty with volar wrist crease hygiene and can yield skin breakdown and infection. Caregivers who dress and clean children with severe wrist flexion deformities are often satisfied with surgeries that place the wrist in a neutral position (Van Heest and Strothman 2009). Aesthetically, a hyperflexed or deformed wrist may be the most noticeable aspect in a child with hemiplegia, and correction of wrist position may increase the child’s social confidence and use in bimanual tasks (Sakellarides and Kirvin 1995).
A variety of procedures have been described to correct wrist deformity in children with cerebral palsy (Green 1942; Green and Banks 1962; Carroll and Craig 1951). Reproducible and successful outcomes depend on the surgeon’s careful preoperative planning. Multiple clinical assessments, goal-directed care, video or functional analysis, interpretation of EMG, identification of dystonia, as well as assessment of voluntary control of the limb and sensibility are all mandatory prior to performing surgery for correction of wrist deformity (Omer and Capen 1976).
Classification
While many classifications of wrist deformity have been described, the Zancolli’s classification (Table 4) considers the composite effect of wrist and finger tightness and can guide surgical management of wrist flexion contractures in children with cerebral palsy.
Table 4
Wrist and finger deformity: Zancolli’s classification
Group 1 | Active finger extension with <20o of wrist flexion |
Group 2 | Active finger extension with more than 20o of wrist flexion |
Group 2a | Active wrist extension with fingers flexed |
Group 2b | No active wrist extension with fingers flexed |
Group 3 | Wrist and finger extension absent even with full wrist flexion |
Positioning
The operating room setup, positioning, and preparation are the same as that described for elbow contracture release and most commonly part of multiple procedures performed at the same setting. Fluoroscopy is used for bone procedures such as arthrodesis and chondrodesis. Setup and trial imaging of the wrist prior to prepping and draping is helpful to plan location and position of the C-arm as well as determine if large C-arm is needed to visualize the entire distal forearm or mini C-arm is adequate. If an elbow flexion contracture release is planned, this should be performed first to allow access to the forearm and wrist.
Surgical Approach
The wrist flexors and extensors can be released, lengthened, harvested, and transferred through various approaches. Options include separate small longitudinal, curvilinear, or transverse incisions (large enough to protect nearby neurovascular structures) or wide longitudinal forearm incisions that expose the entire forearm anatomy. Preoperative marking of all incisions for the forearm, wrist, and fingers is imperative to prevent small skin bridges between incisions.
Wrist Flexor Releases
In mild, dynamic wrist flexion contractures, simple fractional or Z-lengthenings of the wrist flexors can be performed.
Technique: The superficial layer of the flexor-pronator mass can be approached through a long ulnar border incision that is L shaped across the wrist crease or through multiple smaller incisions in the mid forearm. The fascia over each tendon is kept with the volar skin to maintain the integrity of the volar forearm skin. The flexor carpi ulnaris is a workhorse tendon with relatively large power and excursion. Harvesting the tendon from its insertion on the pisiform or lengthening (fractional or Z-lengthening) on the distal tendinous portion requires careful protection of the ulnar artery and nerve just deep to the tendon.
The flexor carpi radialis can be fractionally lengthened effectively within the large tendinous portion of the muscle belly or Z-lengthened more distally in the natural raphe of the tendon. Overlengthening of tendons can be avoided by placing the wrist in the desired extension position and securing arms of the z at that level. Side-by-side repair of the Z-lengthening or weave of the tendon ends can be performed and secured with a nonabsorbable stitch. The palmaris longus tendon can be harvested for transfer, Z-lengthened, or released with a tenotomy at the volar wrist crease.
Tendon Transfer for Wrist Extension
Wrist flexion posturing that is passively correctable and more moderate (30–45°) can be managed with tendon transfers (Beach et al. 1991). If the wrist and finger extensors are weak, then the powerful wrist flexors will be primarily chosen for donors. As these transfers also cross the wrist, FCU or FCR transfers to the EDC can also improve wrist extension. However, asking a tendon transfer to perform more than one task often results in a disappointing outcome. If the finger extensors are present and functioning, the FCU, FCR, ECU, BR, PT, and PL are available for wrist extension transfers. The recipient tendons are either the ECRB or ECRL (Wenner and Johnson 1988). Due to its more radial insertion, insertion into the ECRL will also correct coronal plane deformity. If the ECU is contracted or spastic and contributing to ulnar deviation of the wrist, it should also be transferred more centrally. Preoperative serial clinical exams and EMG testing for phasic, non-phasic, and continuous activity will guide decision making (Kreulen and Smeulders 2008). The ideal wrist transfer will improve wrist and finger extension, permit finger flexion, decrease excessive palmar wrist flexion, and prevent wrist extension deformity. The FCU to ECRB will be described below, but the principles of this technique can be applied to any number of transfers that have been described (Wolf et al. 1998; Thometz and Tachdjian 1988; Patterson et al. 2010; Carroll 1958).
Technique
The FCU is harvested through either a longitudinal ulnar incision or through multiple small transverse incisions. The muscular portion of the tendon extends nearly to the wrist crease and secures the tendon to the ulnar periosteum. This makes harvest through small incisions challenging and frustrating. Through a long ulnar incision, the sheath over the FCU is opened, and the FCU is carefully circumferentially dissected from the ulnar nerve and artery at its insertion into the pisiform and cut transversely as distally as possible. The tendon is then dissected from its ulnar periosteal attachments in a proximal direction while protecting the ulnar neurovascular bundle. A trial of passing the tendon around the ulnar border and over the skin will demonstrate the location of the dorsal incision.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


