Chapter 595 Central Nervous System Infections
Infection of the CNS may be diffuse or focal. Meningitis and encephalitis are examples of diffuse infection. Meningitis implies primary involvement of the meninges, whereas encephalitis indicates brain parenchymal involvement. Because these anatomic boundaries are often not distinct, many patients have evidence of both meningeal and parenchymal involvement and should be considered to have meningoencephalitis. Brain abscess is the best example of a focal infection of the CNS. The neurologic expression of this infection is determined by the site and extent of the abscess(es) (Chapter 596).
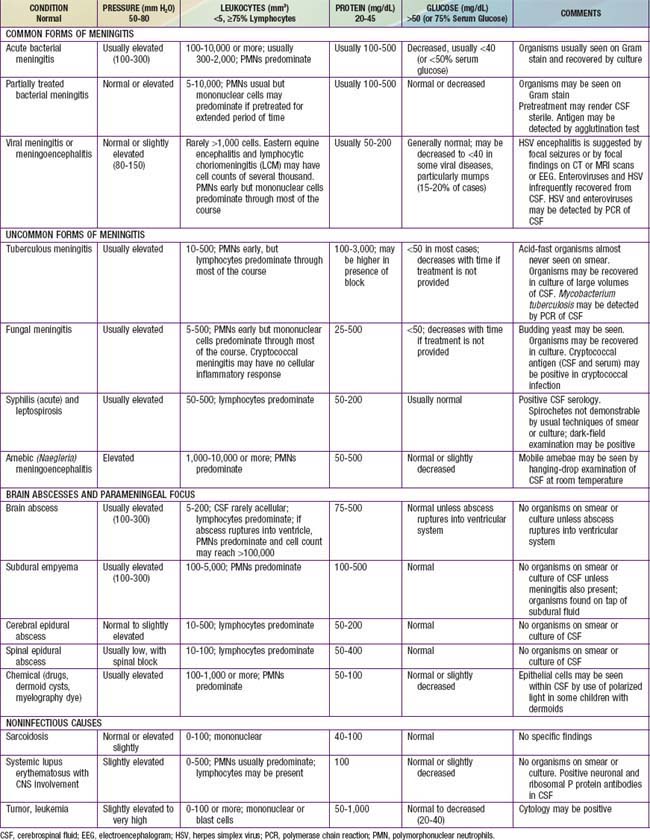
The diagnosis of diffuse CNS infections depends on examination of cerebrospinal fluid (CSF) obtained by lumbar puncture (LP). Table 595-1 provides an overview of the expected CSF abnormalities with various CNS disorders.
595.1 Acute Bacterial Meningitis Beyond the Neonatal Period
Pathology and Pathophysiology
Increased ICP is due to cell death (cytotoxic cerebral edema), cytokine-induced increased capillary vascular permeability (vasogenic cerebral edema), and, possibly, increased hydrostatic pressure (interstitial cerebral edema) after obstructed reabsorption of CSF in the arachnoid villus or obstruction of the flow of fluid from the ventricles. ICP may exceed 300 mm H2O; cerebral perfusion may be further compromised if the cerebral perfusion pressure (mean arterial pressure minus ICP) is <50 cm H2O due to systemic hypotension with reduced cerebral blood flow. The syndrome of inappropriate antidiuretic hormone secretion (SIADH) may produce excessive water retention and potentially increase the risk of elevated ICP (Chapter 553). Hypotonicity of brain extracellular spaces may cause cytotoxic edema after cell swelling and lysis. Tentorial, falx, or cerebellar herniation does not usually occur because the increased ICP is transmitted to the entire subarachnoid space and there is little structural displacement. Furthermore, if the fontanels are still patent, increased ICP is not always dissipated.
Diagnosis
The diagnosis of acute pyogenic meningitis is confirmed by analysis of the CSF, which typically reveals microorganisms on Gram stain and culture, a neutrophilic pleocytosis, elevated protein, and reduced glucose concentrations (see Table 595-1). LP should be performed when bacterial meningitis is suspected. Contraindications for an immediate LP include (1) evidence of increased ICP (other than a bulging fontanel), such as 3rd or 6th cranial nerve palsy with a depressed level of consciousness, or hypertension and bradycardia with respiratory abnormalities (Chapter 584); (2) severe cardiopulmonary compromise requiring prompt resuscitative measures for shock or in patients in whom positioning for the LP would further compromise cardiopulmonary function; and (3) infection of the skin overlying the site of the LP. Thrombocytopenia is a relative contraindication for LP. If an LP is delayed, empirical antibiotic therapy should be initiated. CT scanning for evidence of a brain abscess or increased ICP should not delay therapy. LP may be performed after increased ICP has been treated or a brain abscess has been excluded.
Differential Diagnosis
In addition to S. pneumoniae, N. meningitidis, and H. influenzae type b, many other microorganisms can cause generalized infection of the CNS with similar clinical manifestations. These organisms include less typical bacteria, such as Mycobacterium tuberculosis, Nocardia spp., Treponema pallidum (syphilis), and Borrelia burgdorferi (Lyme disease); fungi, such as those endemic to specific geographic areas (Coccidioides, Histoplasma, and Blastomyces) and those responsible for infections in compromised hosts (Candida, Cryptococcus, and Aspergillus); parasites, such as Toxoplasma gondii and those that cause cysticercosis and, most frequently, viruses (Chapter 595.2) (Table 595-2). Focal infections of the CNS including brain abscess and parameningeal abscess (subdural empyema, cranial and spinal epidural abscess) may also be confused with meningitis. In addition, noninfectious illnesses can cause generalized inflammation of the CNS. Relative to infections, these disorders are uncommon and include malignancy, collagen vascular syndromes, and exposure to toxins (see Table 595-2).
Table 595-2 CLINICAL CONDITIONS AND INFECTIOUS AGENTS ASSOCIATED WITH ASEPTIC MENINGITIS
VIRUSES
BACTERIA
BACTERIAL PARAMENINGEAL FOCUS
FUNGI
PARASITES (EOSINOPHILIC)
PARASITES (NONEOSINOPHILIC)
POSTINFECTIOUS
SYSTEMIC OR IMMUNOLOGICALLY MEDIATED
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree