Cardiovascular Consequences of Obstructive Sleep Apnea
Introduction
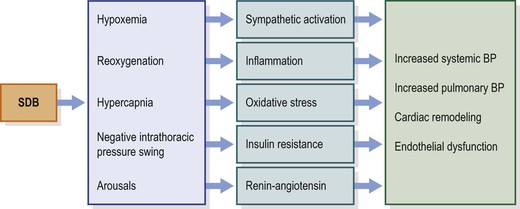
Our knowledge about the link between sleep-disordered breathing (SDB) and cardiovascular dysfunction in children has grown significantly in the last three decades.1–4 Cardiovascular research in children with SDB has primarily focused on disease mechanisms and has been relatively limited on defining the end points that provide unequivocal evidence of cardiovascular morbidity. Nevertheless, small differences in cardiovascular functions between children with SDB and healthy controls have been described in more recent years. The clinical significance of these observations in terms of cardiovascular morbidity either during childhood or in adult life has yet to be delineated. It is also worth noting that the changes in structure and functions of the cardiovascular system are not universally observed across affected populations, with substantial variance occurring in the context of ethnic background, socioeconomic status and body habitus. Therefore, future research that focuses on better understanding the putative contributions of environmental factors, genetic variance, and socioeconomic conditions will be critical to reinforce and explain the observations made to date. This chapter will describe the most recent evidence-based understanding of cardiovascular dysfunction in children with SDB. Proposed hypothetical mechanisms of cardiovascular disease in SDB and evidence of end-organ damage in childhood SDB that have been investigated to date will be discussed (Figure 30-1).
Normal Sleep and Cardiovascular System
Normal sleep is associated with cardiovascular and autonomic changes that exhibit sleep stage dependency.5,6 Progressive reduction of heart rate (HR), blood pressure (BP) and stroke volume during non-rapid eye movement (NREM) sleep occurs synchronously with reduced sympathetic nervous system activity (SNA).7 During REM sleep, sympathetic drive increases markedly with consequent increases in vascular resistance, resulting in elevations of BP and HR.7 Although the exact significance of cyclical and rhythmic alterations in cardiovascular functions during NREM and REM sleep cycle remains unclear, it is believed to be critical for normal cardiovascular health.
Sleep-Disordered Breathing and the Cardiovascular System
Repetitive apneas during sleep disrupt the homeostatic balance of the cardiovascular and autonomic nervous systems.8–10 Increased SNA during episodes of apnea is associated with increased systemic BP, pulmonary arterial pressure and left ventricular (LV) afterload (Figure 30-2).9,11 Hypoxemia and hypercapnia during SDB events are major contributors for chemoreflex-mediated increases in SNA and cardiovascular changes.9 Negative intrathoracic pressures during upper airway obstruction in SDB events affect intrathoracic hemodynamics including LV transmural pressure and LV afterload.12 LV relaxation and LV filling might also be affected by the exaggerated negative intrathoracic pressure swings.13,14 In addition, negative intrathoracic pressure alters aortic pressure, inducing stretch of the aortic wall, activating aortic baroreceptors, and thus buffering sympathetic activation during obstructive apnea.15,16 Upon resumption of breathing, increased venous return distends the right ventricle, reduces LV compliance due to leftward shift of the interventricular septum and thus alters LV diastolic filling.12 Increased stroke volume upon resumption of breathing, at a time when systemic vascular resistance is highest, elicits further increases of BP. SDB events are commonly associated with arousals from sleep, although such might not be as frequent in young children. Nevertheless, arousal may also contribute to the acute increases in BP at termination of SDB events.17 Repetitive nocturnal arousals cause sleep fragmentation that might also be associated with daytime cardiovascular dysfunction.
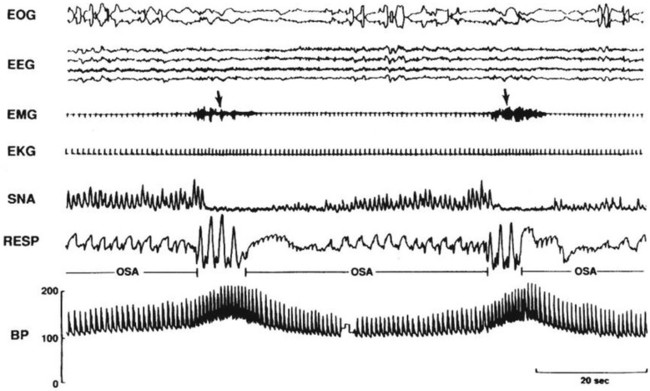
Figure 30-2 Acute effects of apnea during sleep on sympathetic nerve activity (SNA) and BP. Apnea is associated with progressive arterial deoxygenation and sympathetic activation. Resumption of breathing and arousal at the end apnea increases stroke volume and HR and consequent increase in arterial pressure. From Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest 1995;96(4):1897–904.
Mechanisms of Cardiovascular Diseases in SDB
Neural Mechanisms
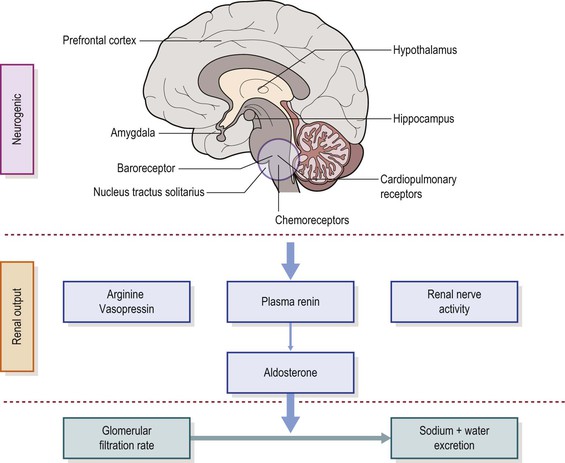
Neural control of the circulation represents the integrated response to diverse reflexes including baroreflex, chemoreflex and low-pressure cardiopulmonary reflexes. The interaction between cardiovascular and respiratory variables constitutes an important influence on neurally mediated changes in cardiac and vascular control (Figure 30-3). Interactions between these reflexes in conditions of hypoxemia and hypercapnia during episodes of OSA are implicated as the primary mechanisms of cardiovascular dysfunction in children with SDB.
Chemoreflex and Cardiovascular System
The chemoreflexes exert profound effects on respiratory, autonomic and cardiovascular functions during apnea events.18 The central chemoreceptors are widely distributed across the central nervous system, are preferentially situated in brainstem regions, and are vital for cardiorespiratory control.19,20 The peripheral chemoreceptors are located within the carotid bodies that respond primarily to hypoxemia and hypercapnia to elicit hyperventilation, tachycardia, and increased sympathetic vasoconstrictor activity.21,22 Activation of peripheral chemoreceptors as a result of cessation of airflow during OSA elicits simultaneous activation of vascular sympathetic and cardiac vagal drives with consequent cardiac slowing and increased BP. Hypercapnia primarily acts through the central chemoreceptors in the brainstem, but also in part through the peripheral chemoreceptors, to produce hyperventilation and increased sympathetic traffic to peripheral blood vessels.23 Interestingly, hypercapnia does not induce vagally mediated bradycardia as seen during hypoxia. Hypoxemia and hypercapnia administered simultaneously induce synergistic increases in minute ventilation.24 Sympathetic activation is also greater during combined hypoxia and hypercapnia than the magnitude of the responses induced by either of the stimuli alone. During OSA, combined hypoxemic–hypercapnic stimuli potentiate the chemoreflex-mediated neural and circulatory responses. Hyperactivity of the peripheral chemoreceptors significantly contributes to the pathogenesis of hypertension in adult patients with OSA.25 In addition, the peripheral chemoreceptors have a greater role in hypoxic responses in young children compared to adults, suggesting that attenuation of peripheral chemoreceptor function develops during maturation.26 Thus, alterations in chemoreceptor-mediated responses in children with SDB and the effects of gas-exchange abnormalities on the developing brain, heart and kidney might be associated with increased risk of cardiovascular morbidity.
Baroreflex and Cardiovascular System
The baroreflex provides critical negative feedback control of arterial pressure.18 Arterial baroreceptors are nerve endings innervating large arteries. Changes in arterial pressure in the aorta and carotid arteries alter the stretch status of the vessels, and result in changes in the frequency of baroreceptor inhibitory impulses to the brainstem cardiovascular centers. Increased baroreceptor activity during a rise in BP triggers reflex inhibition of sympathetic activity, parasympathetic activation, and subsequent decrease in vascular resistance and HR.27,28 Conversely, a decrease in arterial pressure reduces baroreceptor afferent discharge and triggers a reflex increase in sympathetic activity, parasympathetic inhibition and increase in vascular resistance and HR. Thus, reduced baroreflex function may be associated with abnormal control of autonomic and circulatory functions.
Baroreflex and Chemoreflex Interactions
Baroreflex activation inhibits both ventilatory and vasoconstrictor responses to peripheral chemoreflex stimulation.29 Increased BP activates arterial baroreceptors and causes bradycardia.30 Chemoreflex activation in the setting of apnea also elicits bradycardia. The bradycardic response is attenuated when chemoreflex activation and apnea occur in a setting of increased baroreflex activation. Thus, the arterial baroreflex inhibits not only the chemoreflex-mediated sympathetic vasoconstrictor response, but also the vagal bradycardic response.30 The normal buffering influence of the baroreflex may be diminished in patients with SDB,30 resulting in excessive potentiation of chemoreflex sensitivity with consequent exaggerated sympathetic activation and/or brady-dysrhythmias during hypoxemia and apnea. Interaction of chemoreceptor and baroreceptor reflexes during repeated episodes of OSA could be a mechanism for increased BP and promoting hypertension in OSA.31 Decreased baroreflex sensitivity is a potential mechanism of BP elevation in children with SDB.32 Evidence of abnormal baroreflex function in children with SDB will be discussed in the section on SDB-related cardiac dysfunction.
Cortical Modulation of Baroreflex and Chemoreflex
Central command continuously modulates the baroreflex- and chemoreflex-mediated cardiovascular and autonomic functions.33,34 This modulation is important for BP variability during sleep and daytime activities. Several cortical and subcortical brain sites have direct neural projections to the autonomic centers located in the brainstem and modulate their functions. These specific regions in the hypothalamus, amygdala, periaqueductal gray matter and parabranchial nucleus primarily modulate the autonomic drive to the heart and blood vessels to control HR and BP (see Figure 30-3). The interactions between cortical and subcortical brain areas and the brainstem autonomic centers are important for the control of circulatory functions. Intermittent hypoxemia in SDB might affect the CNS integration of baroreflex function, especially in the developing brain of children.
Renin–Angiotensin–Aldosterone System (RAS) Activation
The RAS is important for the regulation of extracellular fluid volume and thus BP (see Figure 30-3).35 Angiotensin II (ANG-II) is a potent vasoconstrictor which acts directly on vascular smooth muscle cells, causing vasoconstriction. ANG-II also regulates blood volume by increasing aldosterone production. Combined effects of ANG-II on vascular resistance and extracellular fluid volume play an important role on BP regulation. Renin released from the kidney is regulated by renal sympathetic nerves. Hypoxia is associated with an increased level of sympathetic nerve and angiotensin II.36,37 OSA patients have higher angiotensin II concentrations compared to healthy subjects.38 Recent studies suggest that resistant hypertension in adults with OSA is associated with activation of the RAS system.39,40 The RAS system also affects 24-hour ambulatory BP in children.35 The role of RAS for BP regulation in children with OSA is poorly understood.
Systemic and Local Inflammation in Sleep-Disordered Breathing
The evidence of increased systemic inflammatory markers in children with SDB is emerging.41 Chronic low-grade inflammation has been linked to the pathophysiology of cardiovascular diseases in SDB.42,43 Patients with OSA have increased levels of interleukin-6, TNF-alpha and C-reactive protein.44 The combination of hypoxemia and sleep deprivation/fragmentation in patients with SDB might lead to increased levels of inflammatory markers. C-reactive protein might itself contribute to vascular disease by inhibiting nitric oxide synthase and increasing cell adhesion molecule expression.45,46 P-selectin, C-reactive protein, fibrinogen, interleukin-8, and interferon levels were found to be higher in children with snoring and/or SDB compared with control subjects. Levels of these cytokines and of circulating adhesion molecules may be reduced by CPAP. A recent study in 143 adolescents reported an elevated level of C-reactive protein in SDB and the levels were directly related to the severity of nocturnal hypoxemia independent of BMI (Figure 30-4).47 Although the majority of the available studies reported significant associations between C-reactive protein serum levels and SDB severity,48 not all studies demonstrated such a relationship.49,50 The conflicting results were in part attributed to the difference in the degree of adiposity and to genetic variance between the study populations.
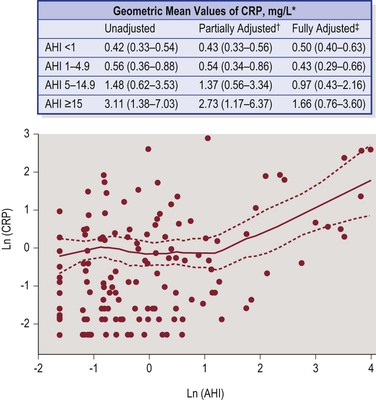
Figure 30-4 Plasma CRP levels in adolescents with OSA and normal controls. Adolescents with OSA, particularly with severe OSA (AHI >5 events/hour) have significantly higher CRP levels. Elevated CRP levels in these patients are directly proportional to the severity of OSA as measured by AHI. *Values are geometric means (95% confidence limits) of CRP (mg/L) values in unadjusted and adjusted models. †Adjusted for age, sex, race. ‡Adjusted for age, sex, race, BMI percentile, (BMI percentile2). From Larkin EK, Rosen CL, Kirchner HL, et al. Variation of C-reactive protein levels in adolescents: association with sleep-disordered breathing and sleep duration. Circulation 2005;111(15):1978–84.
Local inflammation of the upper airway has an important role in modulating airway patency.51 Sputum in children with SDB showed increased neutrophils compared to controls and the level of neutrophil density correlated significantly and positively with the severity of SDB.52 Infiltration of inflammatory cells in the muscular layer of the pharynx has also been described in patients with SDB.53 Active processes of nerve degeneration and regeneration and muscular damage in patients with SDB suggest that inflammation might negatively impact the function of upper airway tissues. However, its role in modulating upper airway collapsibility in patients with SDB has not been fully investigated. Local inflammatory response in the upper airway might be associated with mechanical trauma induced by large intraluminal pharyngeal pressure swings, tissue vibration, and eccentric muscle contraction. Recent studies on the relationship between local inflammation and airway collapsibility suggest an important role of surface tension of upper airway lining fluid for generation of a force which hinders airway opening.54,55 However, the exact mechanisms underlying the up-regulation of inflammatory processes within the upper airway are not clear.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree