Chapter 414 Cardiac Development
414.1 Early Cardiac Morphogenesis
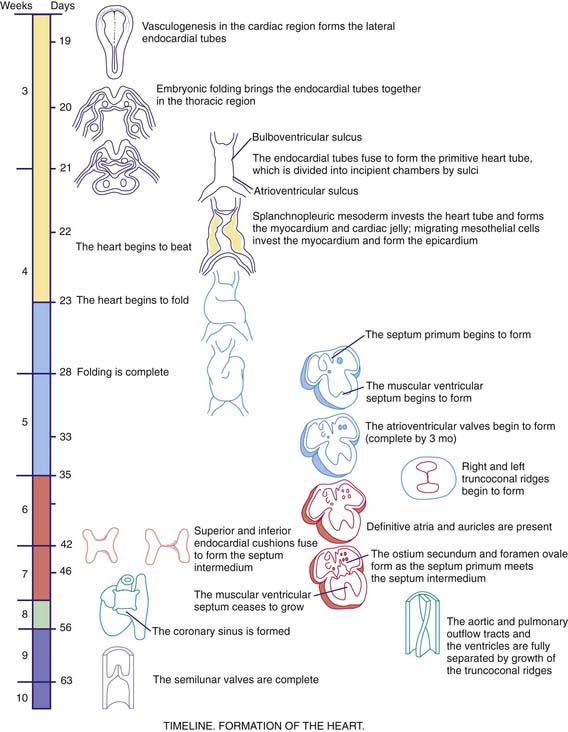
As early as 20-22 days, before cardiac looping, the embryonic heart begins to contract and exhibit phases of the cardiac cycle that are surprisingly similar to those in the mature heart. Morphologists initially identified segments of the heart tube that were believed to correspond to structures in the mature heart (Web Fig. 414-1): the sinus venosus and atrium (right and left atria), the primitive ventricle (left ventricle), the bulbus cordis (right ventricle), and the truncus arteriosus (aorta and pulmonary artery). However, this model is oversimplified. Only the trabecular (most heavily muscularized) portions of the left ventricular myocardium are present in the early cardiac tube; the cells that will become the inlet portion of the left ventricle migrate into the cardiac tube at a later stage (after looping is initiated). Even later to appear are the primordial cells that give rise to the great arteries (truncus arteriosus), including cells derived from the neural crest, which are not present until after cardiac looping is complete. Chamber-specific transcription factors participate in the differentiation of the right and left ventricles. The basic helix-loop-helix (bHLH) transcription factor dHAND is expressed in the developing right ventricle; disruption of this gene or of other transcriptional factors such as myocyte enhancer factors 2C (MEF2C) in mice leads to hypoplasia of the right ventricle. The transcription factor eHAND is expressed in the developing left ventricle and conotruncus and is also critical to their development.
414.2 Cardiac Looping
At ≈22-24 days, the heart tube begins to bend ventrally and toward the right (see Web Fig. 414-1). The heart is the 1st organ to escape from the bilateral symmetry of the early embryo. Looping brings the future left ventricle leftward and in continuity with the sinus venosus (future left and right atria), whereas the future right ventricle is shifted rightward and in continuity with the truncus arteriosus (future aorta and pulmonary artery). This pattern of development explains the relatively common occurrence of the cardiac anomalies double-outlet right ventricle and double-inlet left ventricle and the extreme rarity of double-outlet left ventricle and double-inlet right ventricle (Chapter 424.5). When cardiac looping is abnormal (situs inversus, heterotaxia), the incidence of serious cardiac malformations is high and there are usually associated abnormalities in the L-R patterning of the lungs and abdominal viscera.
414.3 Cardiac Septation
The heart tube now consists of several layers of myocardium and a single layer of endocardium separated by cardiac jelly, an acellular extracellular matrix secreted by the myocardium. Septation of the heart begins at approximately day 26 with the ingrowth of large tissue masses, the endocardial cushions, at both the atrioventricular and conotruncal junctions (see Web Fig. 414-1). These cushions consist of protrusions of cardiac jelly, which, in addition to their role in development, also serve a physiologic function as primitive heart valves. Endocardial cells dedifferentiate and migrate into the cardiac jelly in the region of the endocardial cushions, eventually becoming mesenchymal cells that will form part of the atrioventricular valves.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree