Cancer in Pregnancy
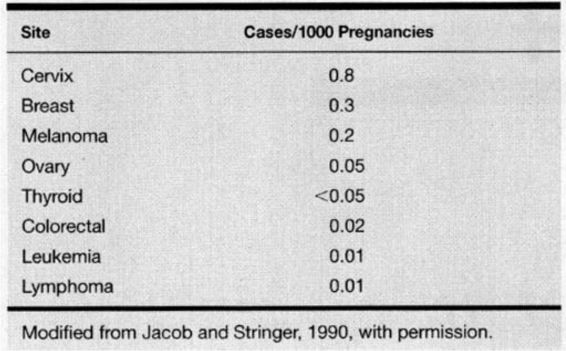
The diagnosis of cancer complicates approximately 1 of every 1000 pregnancies in the United States, and cancer remains the second leading cause of death in women during their reproductive years (Allen and Nisker, 1986; Donegan, 1983; Jacob and Stringer, 1990; Kaiser and associates, 2000). The cancers most commonly diagnosed in pregnancy are listed in Table 24-1. The most frequent cancers during pregnancy are the same as occur in nonpregnant women of the same age. This stands to reason, as there is no data suggesting that pregnancy is either a risk factor for or a protection against any type of malignancy. In recent years, women have tended to delay pregnancy until the third or fourth decade of life. As this trend continues we may see a change in both the incidence and pattern of cancer diagnosed in pregnancy.
TABLE 24-1. Cancers Most Frequently Complicating Pregnancy
The diagnosis of cancer in a pregnant woman creates great emotional turmoil. Regardless of the type of malignancy, therapeutic decision making must consider what is best for the mother and safest for the fetus (Allen and Nisker, 1986; Donegan, 1983; Jacob and Stringer, 1990; Jolles, 1989; Sachs and associates, 1990; Moore and Martin, 1992; Clark, 1991a,b). While a concurrent pregnancy rarely alters the prognosis or treatment of most malignancies, the pregnancy may influence the timing of treatment. Most therapeutic options are associated with risks to the fetus, and frequently it is impossible to develop a treatment strategy that does not compromise optimal treatment of either the cancer or the pregnancy. These complex issues mandate a team approach involving the oncologist, obstetrician, social services, clergy, psychologist, and others, that is individualized to best support the patient and her family. The woman’s final decision regarding therapy will integrate the medical data presented to her with her own ethical and religious beliefs. With improvements in overall patient survival using highly aggressive and toxic treatment regimens, these ethical and emotional issues have become increasingly more complex.
Development of treatment strategies for cancer during pregnancy is compromised by lack of clinical data. Due to the relative infrequency of cancer in pregnancy, the database consists of small retrospective reports, anecdotal descriptions, and editorial reviews. Assuming no change in the incidence of cancer in pregnancy, it is unlikely that prospective data regarding the effects of pregnancy on cancer or the effects of any specific cancer and its treatment on fetal outcome will be available. Our goal within these confines is to summarize available clinical data and provide rational treatment options for those malignancies most frequently encountered in pregnant women (Gilstrap and colleagues, 1996).
DIAGNOSTIC AND THERAPEUTIC MODALITIES
PHYSICAL EXAMINATION
The normal anatomic and physiologic changes of pregnancy can compromise the ability to detect pathologic abnormalities and must be considered when interpreting symptoms and physical findings (Allen and Nisker, 1986; Donegan, 1983; Jacob and Stringer, 1990; Jolles, 1989; Moore and Martin, 1992; Clark, 1991a,b). Many signs and symptoms that would alert the physician to the possibility of malignancy in the nonpregnant patient are frequent events in normal pregnancy, and as such do not merit thorough evaluation. For instance, all pregnant women will experience abdominal distension and bloating sensation at some point in pregnancy. Distinguishing normal from abnormal complaints and discomfort of pregnancy still can lead to dangerous delays in the diagnosis of malignancy (Allen and Nisker, 1986; Donegan, 1983; Jacob and Stringer, 1990; Jolles, 1989; Moore and Martin, 1992; Clark, 1991a,b). Other specific examples of these problems will be illustrated as they pertain to the various malignancies discussed.
DIAGNOSTIC RADIOLOGY
Optimal fetal exposure to radiation is no exposure, yet prudent management of the pregnant patient may necessitate the use of radiographs for prompt diagnosis, as well as for staging and treatment planning after the diagnosis of cancer is made. Some of the anatomic and physiologic changes associated with pregnancy may compromise the value of certain radiologic exams such as mammograms (Allen and Nisker, 1986; Donegan, 1983; Jacob and Stringer, 1990; Jolles, 1989; Moore and Martin, 1992; Clark, 1991a,b). Newer methods of imaging, such as ultrasonography and magnetic resonance, have been proposed as suitable alternatives to x-ray examinations (Kier and associates, 1990).
Radiation-induced damage to the fetus is related to two factors: gestational age at the time of exposure and the radiation dose the fetus receives (Sutcliffe, 1985; Kalter and Warkany, 1983; Timothy, 1986; Brent, 1981; Kinlen and Achejun, 1968; Mole, 1979; Dekaben, 1968; Brill and Forgotson, 1964). The spectrum of radiation effects include fetal death, congenital malformations, developmental and growth retardation, and future malignancy. Radiation exposure at the time of or within 2 weeks of implantation is believed to have an all-or-none effect that may result in embryonic death and abortion (Timothy, 1986). Exposure during the remainder of the first trimester places the fetus at risk for congenital abnormalities, as this is the major period of organogenesis. Radiation exposure during the second and third trimesters has been associated with developmental and growth retardation. The dose of radiation required to induce damage is also related to gestational age. The minimal lethal dose of radiation increases from 0.1 Gy at day 1 of life to greater than 1.0 Gy after the first trimester (Brent, 1981). Doses above 0.1 Gy after the second week of life can result in sublethal complications such as malformations or developmental retardation (Brent, 1981). The risk of these complications increases significantly with increasing radiation dose. The potential for the development of childhood malignancies is present in all three trimesters and can occur with doses as low as .01 Gy (Schlono and colleagues, 1980; Harvey and coworkers, 1985; Sweet and Kinzie, 1976). The embryo is at greatest risk for radiation-induced mental retardation at 8–15 weeks’ gestation, with estimates as low as 4 percent for 0.1 Gy (10 rad) to as high as 60 percent for 1.5 Gy (150 rad) (Committee on Biological Effects, 1990; Hall, 1991). At the same levels of exposure there is a smaller risk at 16–25 weeks’ gestation. There appears to be no significant increased risk of mental retardation at gestational ages less than 8 weeks or greater than 25 weeks, even with radiation exposure exceeding 0.5 Gy (50 rads) (Committee on Biological Effects, 1990).
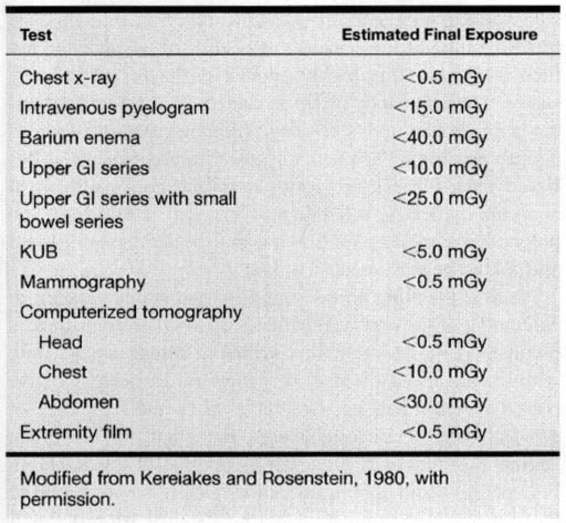
Table 24-2 lists the dose of radiation to the fetus from various diagnostic tests. In general, the levels of ionizing radiation to the fetus from these exams remains significantly below the doses required for potential damage. With multiple exams and exposures there is an additive effect. As pointed out by the American College of Radiology, no single diagnostic test results in a radiation dose significant enough to warrant therapeutic abortion (Hall, 1991).
TABLE 24-2. Radiation Doses Associated with Diagnostic Tests Frequently Used in Oncology
TUMOR MARKERS
Most of the clinically useful tumor markers are of little value in pregnancy as they are normally elevated in pregnancy (ie, carcinoembryonic antigen [CEA], human chorionic gonadotropin [HCG], and α-fetoprotein [AFP]). However, elevations above the normal levels of pregnancy may warrant special attention. Evaluations of extremely high α-fetoprotein levels during pregnancy have yielded the diagnoses of colon cancer, hepatocellular carcinoma, and germ-cell tumors of the ovary (Goldberg and colleagues, 1991; Johnson, 1992; Farahmand and associates, 1991; van der Zee and coworkers, 1991; Gonsoulin and colleagues, 1990; Frederiksen and associates, 1991).
RADIOTHERAPY
Radiation therapy offers the potential for cure in several malignancies. Although the doses of radiation used in cancer therapy are well above the lethal dose for a fetus, use of lead shields and carefully planned dosimetry allow therapeutic doses above the diaphragm and to the extremities without compromising the fetus (Timothy, 1986). Tumors located within the abdomen or pelvis require treatment doses that will result in fetal death.
CHEMOTHERAPY
Advances in chemotherapy have increased longevity and survival in women with cancers previously considered incurable. Chemotherapy is a potentially curative treatment in several malignancies that can affect pregnant women, including ovarian cancer, breast cancer, lymphomas, and leukemias.
Chemotherapeutic agents exert their lethal effects on the most rapidly dividing cells in the body. Because fetal cells are rapidly dividing, chemotherapy poses risks to the fetus, including direct fetal toxicity and/or death, congenital malformations, developmental and growth retardation, and the risk of future malignancy (Kalter and Warkany, 1983; Sweet and Kinzie, 1976; Beeley, 1986; Blatt and colleagues, 1980; Doll and coworkers, 1989; Zemlickis and associates, 1992b; Kim and colleagues, 1992). As with radiotherapy, fetal risks are dependent on the gestational age at which treatment is administered. The major period of risk for anomalies is the first 8 weeks of gestation, corresponding to the major period of organogenesis. Doll and associates (1989) compiled an exhaustive review of the literature and reported a 17 percent incidence of fetal malformation with single-agent chemotherapy and a 25 percent incidence with multiagent chemotherapy when the treatment was administered in the first trimester. With the exceptions of the hematopoietic system, the central nervous system, and the fetal eyes, all other major organ systems are developed by the end of the first trimester and are not at risk for teratogenic anomalies when chemotherapy is administered in the second or third trimester. Correspondingly, Doll and associates (1989) were unable to identify any reports of teratogenicity when single-agent or multiagent chemotherapy was administered in the second or third trimesters. Other factors that affect the risk of chemotherapy include the agents used, frequency and dose of therapy, individual susceptibility, and the number of agents used. Folic acid antagonists are the agents most frequently associated with fetal malformations, while the alkylating agents seem to have less potential for teratogenicity (Doll and associates, 1989). Nonteratogenetic effects (ie, fetal effects) such as growth retardation, premature labor, fetal organ toxicity, and an increased stillbirth rate are associated with antineoplastic agents administered in the second and third trimesters of pregnancy (Sweet and Kinzie, 1976; Blatt and associates, 1980; Doll and coworkers, 1989; Zemlickis and colleagues, 1992b). Data regarding the specific risks of a particular antineoplastic agent is unavailable due to the infrequency of their use in pregnancy. Conclusions regarding the use of these agents in pregnancy must be drawn from theoretical concerns, animal data, and anecdotal reports.
The actual effects of pregnancy on the pharmacokinetics of anticancer drugs remain unknown; however, there are multiple physiologic changes associated with pregnancy that may have a significant effect. These physiologic changes include delayed gastric emptying, which may affect the absorption of oral agents. Increased plasma volume, decreased serum albumin, and a decreased oncotic pressure could affect drug distribution, while an increased glomerular filtration rate and increased hepatic metabolism could potentially increase drug clearance. Additionally, the amniotic fluid may serve as a chemotherapeutic sink. In light of these practical and theoretical concerns, adequate dosing of chemotherapeutic agents is unknown.
SURGERY
Cancers of the cervix, breast, thyroid, and colon, and malignant melanoma have been diagnosed in pregnant women, and are all potentially curable with early surgical intervention. With the exception of cervical cancer, pregnancy rarely alters the surgical management of these patients.
The potential risks to the fetus with surgical intervention include: (a) uteroplacental insufficiency from hypotension, hypoxia, or positioning the patient such that the enlarged uterus compresses the vena cava; (b) preterm labor secondary to peritoneal irritation or uterine manipulation; and (c) infectious complications (Schnider and Webster, 1965; Doll and associates, 1988; Velard, 1984). The risk of teratogenicity of modern anesthetic agents appears to be negligible and not a threat to the fetus (Doll and associates, 1988; Barron, 1984). Uteroplacental insufficiency can generally be avoided with proper positioning of the patient with lateral tilt to displace the uterus from compressing the vena cava, by proper monitoring of maternal oxygenation, and by treatment as indicated with supplemental oxygen and blood and volume replacement (Cunningham and colleagues, 1997; Velard, 1984). Uterine irritability can be avoided by limiting uterine manipulation and the use of copious warm irrigation to remove peritoneal irritants (Schnider and Webster, 1965; Barron, 1984). Although somewhat controversial, the prophylactic use of tocolytic agents during and for 24-72 hours following abdominal surgery may decrease uterine irritability and premature labor. Infectious risks can be kept to a minimum with the appropriate use of antibiotics and careful surgical technique.
Pregnancy has several major effects on surgical intervention; the most obvious effect is the anatomic changes associated with the enlarged uterus, which can compromise exposure during intraabdominal surgery. The pelvic vessels become markedly engorged as pregnancy advances and hemostasis may be difficult to achieve. Pregnancy also increases the risk of thromboembolic complications (Laros and Alger, 1984; Hathaway and Bonner, 1978); all coagulation factors except XI and XIII are increased, fibrinolytic activity is decreased, and lower extremity venous blood flow is more static (Laros and Alger, 1984; Hathaway and Bonner, 1978; Cunningham and colleagues, 1997). Therefore thromboembolic prophylaxis with minidose heparin or sequential compression hose may be indicated in some women.
GYNECOLOGIC MALIGNANCIES
CERVICAL CANCER
Although at one time it was felt that pregnancy had deleterious effects on the outcome and management of cervical cancer, current opinion indicates that, in essence, they are coincidental findings. Although the pregnancy may dictate the timing of therapy, it has no effect on the prognosis or on the fetus. Although carcinoma of the uterine cervix is the most frequently diagnosed cancer in pregnancy, it is still an unusual finding. Method and Brost (1999) reviewed the medical literature in English from 1965 to 1998 on this subject. The overall incidence ranges from 1 to 13 cases in 10,000 pregnancies, although in large referral maternity hospitals, the incidence may be 1 per 1000–2500 deliveries. In large cancer referral centers, about 1 percent of women who have carcinoma of the cervix are pregnant at the time of diagnosis.
Carcinoma of the cervix, irrespective of whether the patient is pregnant, is highly curable if diagnosed in early stages. The pregnancy offers an added opportunity for cancer surveillance and screening. Although several decades ago, an abnormal Papanicolaou smear in pregnancy was believed caused by the pregnancy itself and not by a cervical abnormality, today, reliability of cervical cytology in the pregnant patient is as valid as it is in the nonpregnant patient and should be evaluated accordingly.
Vaginal bleeding is the most common symptom seen in carcinoma of the cervix, regardless of whether the patient is pregnant. Unfortunately, this symptom often appears only with far-advanced disease. As many as 30 percent of reported patients had no symptoms when the diagnosis of cervical cancer was established (Creasman 1970). When vaginal bleeding does occur during pregnancy, investigation is imperative and should not automatically be attributed to the pregnancy. Examination during the first trimester will not lead to an abortion or to premature labor in the third trimester. Added precautions may be necessary because of the pregnancy, but they should not be postponed for fear of interfering with the pregnancy. An incomplete evaluation can lead to a dangerous delay in diagnosis.
In more than half of the asymptomatic patients in one study (Creasman, 1970), the only abnormality noted was that of an abnormal Papanicolaou smear. Colposcopic examination, although usually more time-consuming because of the larger area of the cervix, needs to be evaluated irrespective of the pregnancy. It is just as accurate in the pregnant patient as it is in the nonpregnant patient.
The age range for patients with carcinoma of the cervix spans the reproductive years. Studies have indicated that an age range of 19 to 46 with a mean of 33 years and the age at diagnosis had no influence on the prognosis of the lesion within a given stage. Several reports have noted survival of patients with carcinoma of the cervix associated with pregnancy were similar to that of the nonpregnant patient (Creasman, 1970; Sablinska, 1977; Lee, 1981; Nisken and Shubert, 1983).
In a like matter, parity is not considered a prognostic factor in cervical cancer, although it has been implicated as a causative factor. Although early coital activity appears to be an important etiologic factor, in many instances, early pregnancy and multiparity usually go hand in hand. The average parity for carcinoma of the cervix in the pregnant patient was 5.4. In a similar age group of patients who were not pregnant, the average parity was 3.5 (Creasman, 1970). A more advanced lesion was not found with increasing parity and, therefore, did not influence prognosis.
Time of diagnosis in regards to gestational age may have a major impact upon treatment options. It is therefore imperative to make a diagnosis as early as possible with cervical cytology being a routine part of the initial work-up of a pregnant patient if she has not had one in the recent past. Although it is said that over 90 percent of the adult women in the United States have been screened at least once, only about two-thirds have Papanicolaou smears on a regular basis, which could be every 3 years or more. The guidelines as noted by the American College of Obstetricians and Gynecologists (1995) of yearly Papanicolaou smears should be followed for the pregnant patient as well.
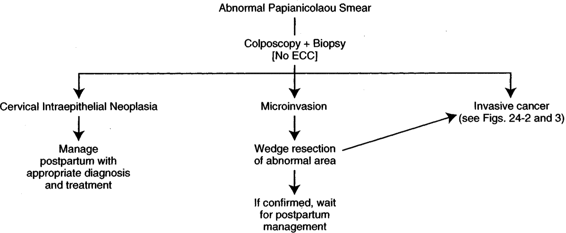
INTRAEPITHELIAL LESION. If a patient has an abnormal Papanicolaou smear as part of her prenatal evaluation, this patient should be evaluated as if she is not pregnant, with some exceptions. Certainly with cytology indicating any level of dysplasia, it is our opinion that these patients should undergo colposcopy. In a recent review of 305 pregnant women with abnormal cervical cytology, Palle and colleagues (2000) concluded that because of a high persistence rate of abnormality, colposcopically directed biopsies should be used liberally during pregnancy. In some instances, performing the colposcopically directed biopsy in the early part of the second trimester is easier than doing it in the first trimester because the pregnancy produces eversion of the cervix, making it easier to evaluate. Because of the increased volume of the everted cervix with its multiple clefts, the process is more time-consuming. Because of the eversion, the ability to see the entire transformation zone is usually quite apparent and the chances of missing an endocervical lesion are minimal. If an abnormality on the cervix is identified colposcopically, we prefer to biopsy this area for documentation. If a physician is involved in the pregnancy management of the patient who has expertise in colposcopy feels comfortable with his or her impression of the likely histology with colposcopy alone, it appears acceptable to not perform a biopsy during pregnancy. It is suggested that in most hands, a biopsy should probably be taken (Palle and colleagues, 2000). Certainly, the pregnant cervix is more apt to bleed than is the nonpregnant cervix, but the bleeding is usually controllable with Monsel solution, and it is rare that a suture is required to stop the bleeding. Endocervical curettage (ECC) is not performed. If only intraepithelial disease is noted on cytology, colposcopic evaluation, and biopsy, this patient can be followed through her pregnancy, be allowed to deliver vaginally, and be managed postpartum in the routine fashion (Fig. 24-1). We prefer to obtain one or two subsequent Papanicolaou smears as a precautionary basis during pregnancy, depending upon when the initial evaluation is performed. It has not been our practice to repeat colposcopy. Progression during pregnancy to invasive cancer has not been encountered. If, on the other hand, cytology, colposcopy, or biopsy suggests a microinvasive lesion, further evaluation is required. Some authors suggest that this is the only indication for conization in pregnancy (as in the nonpregnant patient). Classic cone in pregnancy can have a disastrous effect, with significant bleeding and even a loss of the pregnancy. We find that a “wedge” of the affected portion of the cervix gives the necessary information with much less chance of having significant side effects. Injection of the lesion with a dilute solution of Pitressin Synthetic (vasopressin) also helps with hemostasis. If <3 mm of invasion is present with clear surgical margins, this patient could be handled much as the intraepithelial lesions. The patient can be followed through the pregnancy expectantly, and there is no contraindication to vaginal delivery. In many instances, this may be adequate treatment, particularly if the patient is desirous of future fertility and more definitive treatment postpartum is not indicated. One may want to do a classic cone postpartum before that final decision is made. A simple hysterectomy as definitive therapy is usually all that is indicated in these patients. On the other hand, if a greater depth of invasion is noted on the wedge or conization, then that patient may need to be managed more aggressively. If, in fact, conization is done during pregnancy, it should be “flat” instead of the classic cone.
FIGURE 24-1. Management of abnormal Papanicolaou smear in pregnancy.
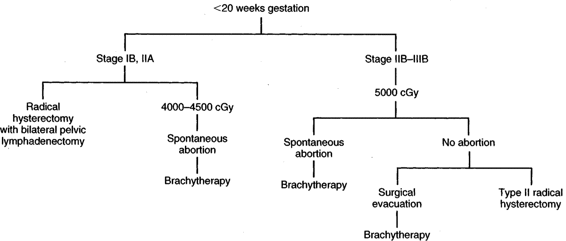
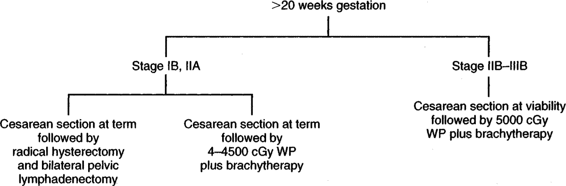
INVASIVE CARCINOMA. Although there are no hard-and-fast rules concerning age of gestation and treatment, it is accepted that if a patient is <20 weeks gestation, then definitive therapy for the cancer should be performed and the pregnancy ignored. There are obvious exceptions to this depending upon the patient’s desires and the risk she is willing to take. In early stage disease (IB-IIA), unless there are contraindications, radical hysterectomy and pelvic lymphadenectomy are the treatment advocated by many authors (Monk, 1992; Sivanesaratnam, 1993; and their colleagues). Whether hysterotomy for technical reasons is necessary prior to definitive surgery, the clinical decision is made intraoperatively. Radiation therapy, as in the nonpregnant patient, appears to be just as efficacious as radical surgery. External radiation can commence and the pregnancy is usually aborted by 3–4000 cGy; then brachytherapy can be applied at completion of the external irradiation. In a small number of patients, abortion will not have occurred prior to the completion of external radiation, and evacuation of the uterus will be required (Fig. 24-2). After 20 weeks gestation, allowing the patient to go to term before definitive therapy for her cervical cancer is an option, realizing that there may be as much as 3 months or longer delay before primary treatment of this cancer. A decision in regards to optimal time of delivery must be made in concert with the maternal-fetal specialist. Although data suggest that the route of delivery, even in cases of clinical cervical cancer, has no impact on either the mother or the fetus, most authors suggest cesarean section as the preferred mode of delivery. At least theoretically, there appears to be a lesser chance of bleeding from trauma of the cervix dilatating or even failure to dilatate. In early stage disease, a cesarean section followed by radical surgery could be performed at the same setting with no more morbidity than doing the surgery in the nonpregnant patient (Fig. 24-3). In advanced disease (IIB or greater), standard radiation therapy is given (Baltzer, 1990; Hannigan, 1990, Hopkins, 1992; and their colleagues). External therapy can commence immediately postoperatively. It should be noted that reports of episiotomy site recurrence of squamous cell carcinoma of the cervix have been noted (Burgess, 1987; Copeland, 1987; and their colleagues). Although this is a rare site of recurrence, it has occurred even in early stage disease, and careful monitoring of the perineum in the immediate postoperative period appears to be efficacious.
FIGURE 24-2. Management of invasive cervical carcinoma less than 20 weeks gestational age.
FIGURE 24-3. Management of invasive cervical carcinoma greater than 20 weeks gestation. WP = whole pelvis.
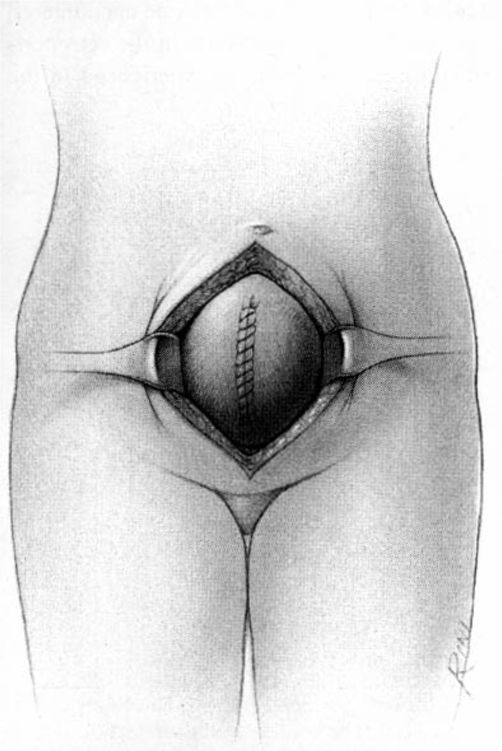
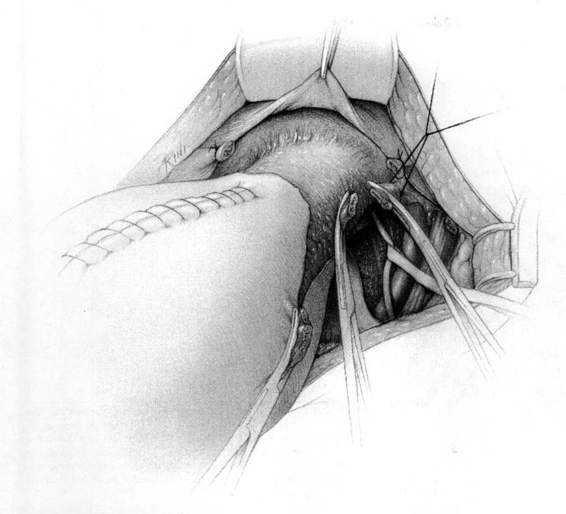
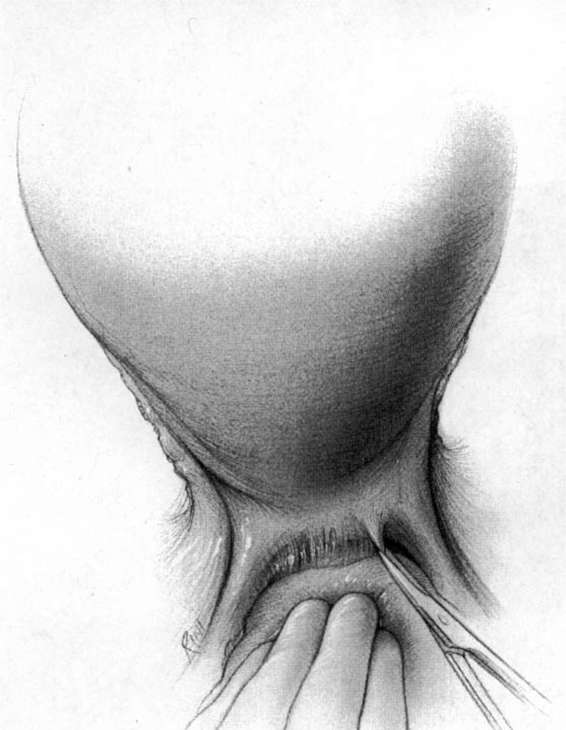
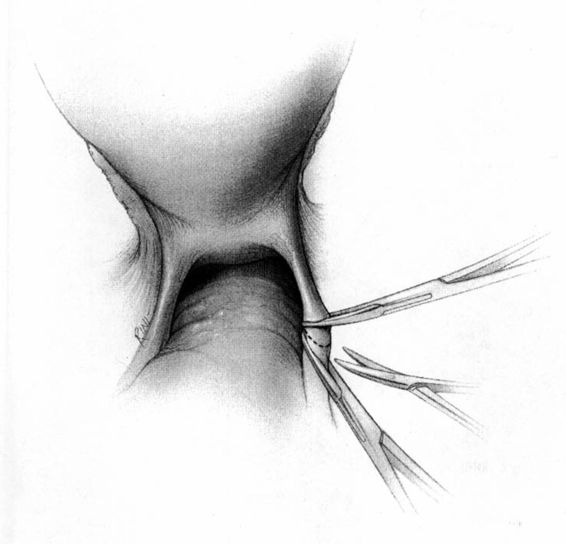
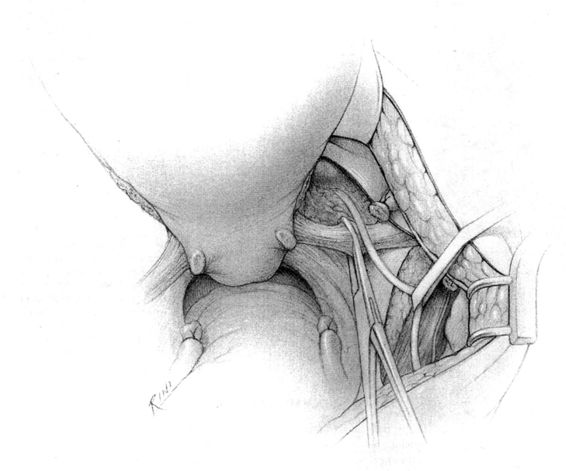
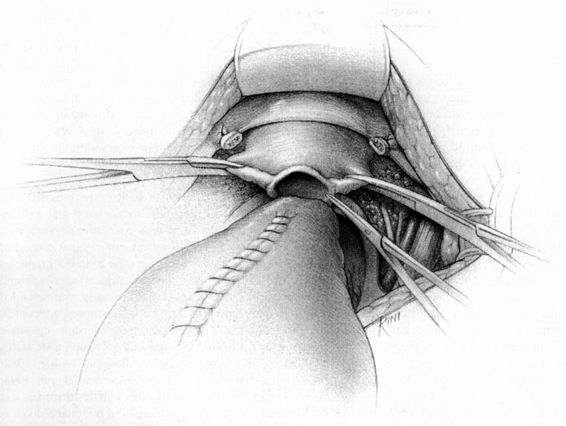
It should be noted that even with more pregnant patients seeking care earlier in their gestation and cancer surveillance performed, nevertheless many authors note that a considerable number of patients will have their cancer diagnosed in the third trimester or even postpartum. It was surprising to note that patients with advanced lesions were diagnosed 3–6 months postpartum after a normal vaginal delivery without the diagnosis being made intrapartum or had no unusual complications. The technique of radical hysterectomy following classical cesarean delivery is summarized in Figures 24-4 through 24-11.
FIGURE 24-4. After delivery of the fetus through a classic cesarean section incision, the uterus is closed with a single running suture to increase exposure and decrease blood loss.
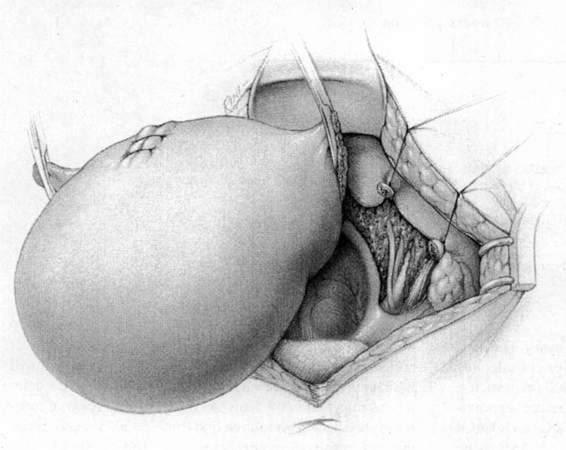
FIGURE 24-5. The round ligaments are then sacrificed close to the pelvic sidewall and the retroperitoneal spaces are opened. The ureters, pelvic vessels, and infundibulopelvic ligaments are clearly identified, and the adnexa are separated from the uterus and reflected cephalad by incising the peritoneal to allow for their preservation. The ureter is then carefully dissected from its attachment to the medial leaf of the broad ligament using sharp dissection.
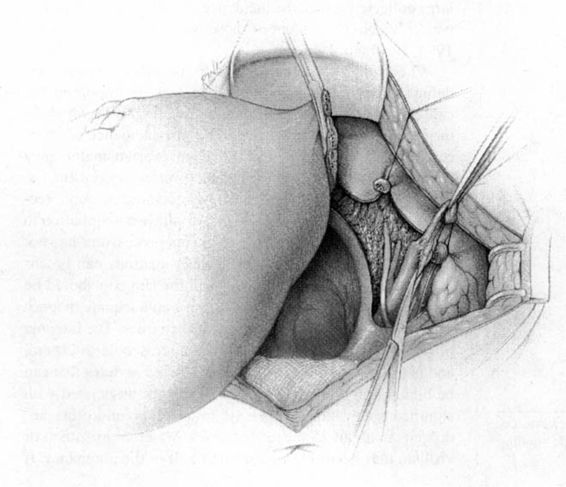
FIGURE 24-6. The pelvic lymphadenectomy is then accomplished. The borders of this dissection are the genital femoral nerve laterally, and the superior vesical artery medially (not shown), the obturator fossa in the caudad direction, and the bifurcation of the aorta in the cephalad direction.
FIGURE 24-7. The bladder is sharply dissected free of its attachment to the lower uterine segment and the upper one-third to one-half of the vagina. The ureter is sharply dissected free and removed from the vesicouterine ligament. The uterine artery is identified and sacrificed at its origin. Each of these steps results in removal of far more parametrial tissue than occurs at the usual hysterectomy.
FIGURE 24-8. Posterior dissection involves separating the uterus and upper one-third to one-half of the vagina from its attachments to the rectum. Shown is initiation of sharp dissection of the posterior peritoneal reflection. This will allow the rectum to be either bluntly or sharply dissected off of the lower uterine segment and posterior vaginal wall and minimize the risk of injury to the rectum.
FIGURE 24-9. The ureterosacral ligaments are transected at their origin, well away from their uterine insertion site. Shown is use of a Heaney- or Roger-type clamp to doubly clamp the uterosacral ligament, following which it is severed between the clamps with Mayo scissors. The distal portion of the ligament is then secured with a transfixing suture.
FIGURE 24-10. The cardinal ligaments are then transected at their origin on the pelvic sidewall.
FIGURE 24-11
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree