Bronchopulmonary Dysplasia
Kathleen A. Kennedy
Joseph B. Warshaw
Most neonates with acute lung disease recover completely within the first week of life. Some of these infants, however, develop chronic respiratory symptoms that persist for weeks to years. In 1967, Northway and colleagues first described the clinical, radiologic, and pathologic manifestations of chronic lung disease in survivors of hyaline membrane disease (HMD) and introduced the term bronchopulmonary dysplasia (BPD). As neonatal intensive care has become more sophisticated over the past three decades, the survival of very low-birth-weight infants has increased dramatically, and chronic lung disease is now seen in very small infants who did not have significant lung disease in the first few days of postnatal life. Although the birth-weight–specific incidence of BPD seems to have remained fairly stable with increasing survival rates, the result of increased survival has been an increase in the absolute numbers of survivors with BPD. In a population-based study of infants born in North Carolina in 1994, chronic lung disease (CLD), defined as either ventilator or oxygen requirement at 30 days postnatal age or 36 weeks postmenstrual age, occurred in 44% and 25% of 500- to 1,500-g survivors, respectively. In the National Institute of Child Health and Human Development (NICHD) Neonatal Research Network, for 501- to 1,500-g infants born in 1995 to 1996, the incidence of CLD (defined as oxygen use at 36 weeks postmenstrual age among 36-week survivors) ranged from 3% to 43% among the centers. The incidence was 52% in 501- to 750-g infants, 34% in 751- to 1,000-g infants, 15% in 1,001- to 1,250-g infants, and 7% in 1,251- to 1,500-g infants. In a variety of study populations, lower birth weight, white race, and male sex have been consistently identified as risk factors for the development of BPD.
DEFINITION
In 1979, Bancalari characterized BPD as tachypnea, retractions, and supplemental oxygen requirement for more than 28 days in infants who had received positive-pressure ventilation for at least 3 days in the first week of life. Associated chest radiograph findings included strandlike densities in both lung fields alternating with areas of normal or increased lucency. The term CLD, usually defined as supplemental oxygen administration at either 28 days of age or 36 weeks adjusted postmenstrual age, has been commonly used in multicenter studies largely for pragmatic reasons. The more stringent diagnostic criterion of oxygen therapy at 36 weeks corrected postmenstrual age has been recommended, because this definition was shown to be a more specific predictor of long-term pulmonary morbidity in very low-birth-weight infants when compared with oxygen therapy at 28 days. Definitions based on oxygen therapy alone have been criticized because many infants have variable requirements for oxygen over time and because the use of supplemental oxygen depends on the oxygen saturation goals chosen by the caregiver. In a recent National Institutes of Health–sponsored workshop on BPD, use of the older term BPD was recommended to maintain a distinction from chronic lung diseases occurring later in life. A new definition of BPD was proposed to distinguish differences in disease severity (mild, moderate, and severe) (see Table 50.1).
TABLE 50.1. NICHD/NHLBI WORKSHOP DEFINITION OF BPD | ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||
ETIOLOGY AND PATHOPHYSIOLOGY
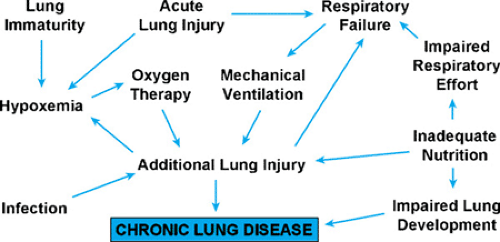
Oxygen toxicity and barotrauma/volutrauma have been implicated in the pathogenesis of BPD, but it is very difficult to isolate these factors from the pulmonary immaturity and acute lung injury for which these treatment modalities are used (see Fig. 50.1). Although BPD initially was described as a complication of HMD [now called respiratory distress syndrome (RDS)], chronic lung disease has become a significant problem in very premature infants without acute lung disease. Conversely, full-term infants who require aggressive oxygen
and ventilator therapy for meconium aspiration, congenital pneumonia, or persistent pulmonary hypertension infrequently develop BPD. Pulmonary air leak, pulmonary edema, patent ductus arteriosus, acquired pneumonia, and poor nutrition also are risk factors for the development of BPD, but these are complications of acute lung injury and prematurity, so their causal role in the pathogenesis of BPD is difficult to establish. For a variety of reasons, the premature lung seems to have an increased susceptibility to iatrogenic lung injury or a decreased capacity to undergo a normal healing process when lung injury occurs. Genetic factors predisposing an infant to develop BPD are suggested by an increased incidence of asthma in first-degree relatives of infants with prolonged BPD.
and ventilator therapy for meconium aspiration, congenital pneumonia, or persistent pulmonary hypertension infrequently develop BPD. Pulmonary air leak, pulmonary edema, patent ductus arteriosus, acquired pneumonia, and poor nutrition also are risk factors for the development of BPD, but these are complications of acute lung injury and prematurity, so their causal role in the pathogenesis of BPD is difficult to establish. For a variety of reasons, the premature lung seems to have an increased susceptibility to iatrogenic lung injury or a decreased capacity to undergo a normal healing process when lung injury occurs. Genetic factors predisposing an infant to develop BPD are suggested by an increased incidence of asthma in first-degree relatives of infants with prolonged BPD.
Pulmonary edema and alveolar necrosis develop within 2 to 3 days in healthy mammals exposed to normobaric hyperoxia. This acute injury is followed by a chronic phase that is pathologically similar to BPD and is characterized by interstitial fibrosis with proliferation of alveolar type II cells and fibroblasts. A causal role of oxygen toxicity is supported by observations in the STOP-ROP study in which infants treated with higher FIO2 to maintain higher O2 saturation goals had worse pulmonary outcomes. Protection from the toxic effects of oxygen seems to be related to the ability to prevent or repair cellular damage caused by oxygen free radicals; a variety of enzymatic and nonenzymatic antioxidants are involved in this protection. The premature neonate may be relatively deficient in some of these antioxidants, but there have been relatively few trials of antioxidants in human infants to prevent BPD.
Intratracheally administered superoxide dismutase may decrease the long-term severity of BPD, although it does not appear to affect the incidence of BPD. This finding is provocative and deserves further study. Vitamins A and E are nonenzymatic antioxidants that prevent free radical propagation in cell membranes. Although vitamin E deficiency exacerbates pulmonary oxygen toxicity in laboratory animals, pharmacologic doses of vitamin E do not afford additional protection in nondeficient animals or humans. Neonates have low vitamin E stores at birth and are at risk for deficiency if vitamin E is not provided enterally or parenterally. Deficiency states should be preventable with the use of early parenteral nutrition and fortified human milk or preterm formulas. Premature neonates of less than 36 weeks gestation also have low plasma concentrations and tissue stores of vitamin A. Supplementation with intramuscular vitamin A has been shown to increase the likelihood of survival without CLD in infants less than 1,000 g birth weight requiring supplemental oxygen or mechanical ventilation at 24 hours of age.
Deficiencies of sulfur-containing amino acids and trace minerals such as selenium increase the susceptibility to oxygen-induced lung injury in animals. Such deficiencies are unlikely to occur in infants who are receiving standard enteral formulas for preterm infants. Less is known about the optimal amounts of specific amino acids and trace minerals that should be supplied in parenteral nutrition solutions. Despite evidence from animal studies that pulmonary oxygen toxicity could be ameliorated by polyunsaturated fatty acid supplementation, preliminary studies in preterm human infants have shown no benefit.
A causal role of barotrauma/volutrauma in the pathogenesis of BPD is supported by the following observations. BPD was seen rarely before positive-pressure ventilators came into use, and some infants develop BPD after mechanical ventilation with low oxygen concentrations. Acute manifestations of barotrauma (pulmonary interstitial emphysema and pneumothorax) are frequent precursors of BPD. Although many investigators have attempted to reduce barotrauma/volutrauma by using different strategies with conventional mechanical ventilators, there have been no large trials to evaluate the impact of particular styles of conventional mechanical ventilation on BPD. Several large trials of synchronized versus nonsynchronized ventilation have shown no effect on BPD. Studies of high frequency oscillatory ventilation to prevent BPD have had variable results; a meta-analysis of all randomized trials showed a modest reduction in CLD that must be balanced against the adverse effects on long-term neurologic outcome shown in the only large trial that assessed long-term outcomes. Permissive hypercapnia (maintaining the PaCO2 at greater than 45 to 50) in mechanically ventilated infants has been variably successful in preventing CLD and deserves further study.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree