Breastfeeding and the Use of Human Milk in the Neonatal Intensive Care Unit
Kathleen A. Marinelli
Kathy Hamelin
OVERVIEW
Over the past two decades, as advances in technology have markedly improved our success in neonatal medicine, we have concomitantly recognized that nutrition is the cornerstone of the care we provide to sick and preterm neonates. The introduction and refinement of total parenteral nutrition, and the development of specialty premature enteral formulas have paralleled these improved outcomes. Over this same time period, there has also been an increasing awareness by both the general public and the medical community of the short- and long-term advantages of both human milk and breastfeeding. Based on a rapidly increasing body of research, there is no question that human milk is uniquely superior to other forms of nutrition for infants. The most recent American Academy of Pediatrics (AAP) policy statement on the use of human milk reminds us that the breastfed infant is the reference or normative model against which all alternative feeding methods must be measured regarding growth, health, development, and other short- and long-term outcomes (1). This position is endorsed and echoed by the American College of Obstetrics and Gynecology (2), the American Academy of Family Physicians (3), the American Dietetic Association (4), and the Canadian Pediatric Society (5). The evidence for the advantages of human milk to not only babies, but mothers, families and society in such diverse areas as health, nutrition, development, and immunology, with psychological, social, economic, and environmental impact (1,2,3,4) is so compelling that the U. S. government has made the support and promotion of breastfeeding a national public health priority. With the release of the Surgeon General’s HHS Blueprint for Action on Breastfeeding (6) and the United States Breastfeeding Committee’s Breastfeeding in the United States: A National Agenda (7) the federal government has embraced the Healthy People 2010 breastfeeding goals of 75% initiation, 50% breastfeeding at 6 months, and 25% breastfeeding at 1 year (8). The June 2004 launching of a 3 year, $40 million National Breastfeeding Awareness Campaign by the U.S. Department of Health and Human Services, Office of Women’s Health, with the tag line “Babies were born to be breastfed” exclusively for 6 months, is clear indication of the commitment to breastfeeding promotion and support (9). Produced in conjunction with the Ad Council, whose previous credits include “Only you can prevent forest fires,” “A mind is a terrible thing to waste,” and “Friends don’t let friends drive drunk,” these Public Service Announcements will target the general market, particularly first time parents, and the African American community, as rates of breastfeeding are lowest among this population (10).
In the ideal scenario, a discussion of breastfeeding generally conjures up a Madonna-like picture of a robust, healthy term newborn, eagerly latched and nursing well with a mother who has had an uncomplicated delivery and is supported and empowered by her ability to continue to nourish her baby. However, the reality of the neonatal intensive care unit (NICU) is often in stark contrast to this. Are the uncertainty, the stress, the technology, the constantly changing and critical nature of our patients and our
environment incompatible with the concept of breast-feeding? On the contrary, for these most vulnerable of our patients and their families, the exponentially increasing body of research supports that both the provision of human milk and breastfeeding are not only as important as they are in the full term population, but in fact may be more critical to the ultimate health and developmental outcome of these sick and premature babies. The AAP policy statement specifically recommends human milk as the preferred feeding not only for healthy term infants, but for sick and premature infants as well (1).
environment incompatible with the concept of breast-feeding? On the contrary, for these most vulnerable of our patients and their families, the exponentially increasing body of research supports that both the provision of human milk and breastfeeding are not only as important as they are in the full term population, but in fact may be more critical to the ultimate health and developmental outcome of these sick and premature babies. The AAP policy statement specifically recommends human milk as the preferred feeding not only for healthy term infants, but for sick and premature infants as well (1).
It is incumbent on us as practitioners to have the knowledge and the expertise to promote and successfully support lactation in the NICU population. Unfortunately, most of us received a cursory education on lactation, if at all, during medical school, residency and fellowship training. The same is true of nursing, nurse practitioner and physician assistant training. This is evidenced by the lack of physician knowledge of and confidence in the subject (11,12,13), the variable and non-evidence-based information present in current general pediatric textbooks (14) and the paucity of neonatal textbooks that even include it. This chapter presents our current knowledge of the unique benefits of the use of human milk in a preterm and sick NICU population, supporting an evidence-based rationale for its important role in our therapeutic regimen. It will also detail the challenges to the provision of human milk, including the decision to express human milk and breastfeed, initiating and maintaining lactation with a breast pump, breast milk supply, the use of donor human milk, the developmental progression toward breastfeeding, the use of alternative feeding methods, and supporting breastfeeding in the NICU and after discharge.
BACKGROUND
Prior to the advent of NICUs and the technology that has made them possible, most premature infants did not survive. Those that were developmentally and physiologically mature enough did so if they could be kept warm, and nourished. The source of that nourishment was human milk. It would then not be an unreasonable leap of faith to say, that until this past century, the survival of premature infants was in large part dependent on the provision of human milk. As early as 1907, Pierre Budin, at L’H¯pital Maternité in Paris, encouraged mothers of premature infants to breastfeed to improve survival (15). Julius Hess, who in Chicago began the first continuously operating center for premature infants in the United States, wrote in 1922 “by far the best results are obtained in the premature infant weighing less than 1500 grams when it is fed human milk” (16). He advocated that human milk was the choice for feeding premature infants, with artificial milk a poor substitute, resulting in increased mortality. It is astounding that at that time there was even positive discussion about the survival of very low birth weight babies, let alone the association of improved survival with human milk feedings!
So why are we just now “re-discovering” the value of human milk in the neonatal unit? In 1947 Gordon showed that premature infants fed two different formulas based on bovine milk gained weight faster than infants who were fed human milk (17). It had also been previously shown that human milk did not support bone mineralization in premature infants unless supplemented with calcium and phosphorus (18). Based on studies like these, the use of human milk was abandoned in the United States for formulas that provided higher protein and mineral intakes. Although the latter was an important observation that needs to be considered today, what was not realized at the time, was how much was lost to obtain this gain.
INCIDENCE OF BREASTFEEDING OR PROVISION OF HUMAN MILK
Until very recently (19,20), the only on-going large-scale source of breastfeeding data in the United States has been the Ross Laboratories Mothers Survey (21,22), which was developed in 1954 and is periodically updated. Ross Products is a division of Abbott Laboratories, a manufacturer of infant formulas. Surveys are mailed to large numbers of mothers who have given birth within the previous year. Included among concerns of using this data to define breastfeeding rates and goals in the United States are low response rates (average 28% per month since 1997) (21), inability to determine exclusivity of breastfeeding, no differentiation between breastfeeding and breast milk feeding, and conflict of interest in a formula company monitoring breastfeeding rates. Although the population of NICU babies or premature babies per se are not analyzed, data is reported on the subset of babies with birth weights less than 2,500 grams, which would combine both premature babies and small for gestational age term babies. For 1990 the in-hospital breastfeeding rates in this weight category are given as 36.5% (compared to overall population breastfeeding rate of 51.5%) with steady increases to 62.7% (compared to overall increase to 69.5%) in 2001 (21,22). In comparison, recently published data from the Centers for Disease Control and Prevention (CDC), using questions added to the National Immunization Survey for the third quarter of 2001, showed a similar overall U.S. initiation of 65.1% (95% CI: 59.5%-70.7%) (19). The CDC has also looked at breastfeeding in 10 states using the Pregnancy Risk Assessment and Monitoring System (PRAMS). For 1993 they report 57% overall initiation, with 40.3% for babies less than 2500 grams, and 49.4% for babies admitted to the NICU. This improved in 1998 to 67.5% overall initiation, 57.9% low birth weight initiation, and 64.3% NICU initiation (20). These data, although less commercially biased, are still difficult to interpret and compare to other studies as a result of inconsistency of definitions. The definition of initiation of breastfeeding varies between studies, and often includes any attempt at putting a baby to breast or initiating lactation with a breast pump in the first several
days of life or during the initial hospitalization. It also does not indicate whether other liquids are given at that time.
days of life or during the initial hospitalization. It also does not indicate whether other liquids are given at that time.
These same reports have tried to look at duration of breastfeeding by surveying for any breastfeeding activity later in the first year of life. The Ross Mothers Surveys report continued breastfeeding at 6 months in babies less than 2,500 grams birth-weight increasing from 9.5% in 1990 to 22.1% in 2001 (with concomitant changes in the entire population of 17.6% to 32.5%) (21,22). This data looked at babies with “any breastfeeding”; including only breastfed, breast milk/breastfeeding mixed with formula feedings (no quantification determined), and only occasional breast milk/breastfeeding (again no quantification). For 2001 they added the category of “exclusive” breastfeeding, reporting for all infants in-hospital as 46.3%, falling to 17.2% at 6 months; for babies less than 2,500 grams, 27.1% in-hospital falling to 8.4% at 6 months (21). Exclusive was defined as “fed only human milk; no supplemental formula and/or cow’s milk.” No information on solid foods or other non-formula supplements (e.g., water, juices) fed to infants were collected (21). The PRAMS study looked at “predominant” breastfeeding at 10 weeks postpartum and showed decreases between 1993 to 1998 of 58.5% to 57.9% in the entire population, 47.9% to 45.1% in the low birth weight subgroup, and 55.1% to 47.3% in the NICU subgroup (20). When duration data is given, it has historically been any breastfeeding or breast milk consumption, with no differentiation made for whether other liquids or solids are ingested and in what quantities. This issue was addressed in 1988 by the Interagency Group for Action on Breastfeeding, who developed a set of definitions to standardize terminology. In the system they describe, full breastfeeding is distinguished from partial breastfeeding, with full subdivided into categories of exclusive and almost-exclusive breastfeeding, and partial differentiated into three levels (23). The hope was that consistent widespread implementation of these definitions would assist researchers and agencies to describe, interpret and compare breastfeeding practices accurately. Clearly, this has not occurred.
In March 2004, the Breastfeeding Committee for Canada issued a document on breastfeeding definitions. They used work previously done in this area, including the definitions from the Interagency Group for Action on Breastfeeding, to develop definitions in an algorithmic format that will facilitate data collection that is consistent, and can be used to compare breastfeeding practices between Canadian provinces and territories (Table 23-1) (24). These definitions are well thought out, and clearly delineate the amount of human milk vs. other liquids that are being consumed. The one thing they do not do, however, is to separate out breast milk feedings from breastfeeding.
When attempting to elucidate trends in breastfeeding and human milk consumption specifically in a NICU population, one can examine a number of reports from individual NICUs. For example, in one author’s NICU (KM), breast-feeding rates have been tracked over a 15-year period. During this time, breast-feeding promotion and support has been greatly increased (which will be detailed later in this chapter). In the entire NICU population, only 20.2% of babies received any human milk (either by breastfeeding or alternative feeding method) in 1989, which steadily increased to 70.8% in 2002. Continuing to receive any human milk at discharge increased from 5.9% to 55.9% of all NICU discharges over this same time period. Even more striking is the data for the population less than 1,500 grams: initiation increased from 10.6% to 82.6% with continued provision of any human milk at discharge from 1.6% to 38.2% (25).
TABLE 23-1 BREASTFEEDING DEFINITIONS | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||
Although the numbers vary, overall trends show an increase in initiation and continuation of breastfeeding activity over the past decade, with significantly fewer babies in low birth weight or NICU categories than healthy, full term babies breastfeeding at any time point. Single institutional reports from the United States and Canada have substantiated that NICU breastfeeding rates are lower than in their equivalent well-baby populations (26,27,28,29), with rapid attrition over the course of the hospitalization and after discharge (29,30,31,32,33). Factors often associated with continued lactation in this population are mothers who are older, married, of Caucasian race, with more than a high school education, a good social support system and having babies with increasing birth weight (27,30,33,34,35). An interesting recent multicenter study by Powers and associates used an administrative database to look at breastfeeding in 42,891 neonates admitted to 124 NICUs from January 1999 to December 2000 (36). They show that 50% of neonates discharged home from
NICUs are not receiving human milk, and they confirm that greater birth weight, older gestational age, white race, increasing maternal age, and married parents are associated with increased likelihood of receipt of at least some human milk at discharge. Of note, they also showed that site of care is a significant independent factor associated with human milk use. This raises questions regarding site differences that are more or less likely to promote successful breastfeeding in an intensive care setting.
NICUs are not receiving human milk, and they confirm that greater birth weight, older gestational age, white race, increasing maternal age, and married parents are associated with increased likelihood of receipt of at least some human milk at discharge. Of note, they also showed that site of care is a significant independent factor associated with human milk use. This raises questions regarding site differences that are more or less likely to promote successful breastfeeding in an intensive care setting.
It is also important to note that these trends in North American NICUs are not necessarily replicated in other parts of the world. As early as the 1980s, reports from European (37,38,39), Brazilian (40), and Australian (41) units demonstrate breastfeeding initiation rates, duration, and eventual exclusivity in premature babies that are equal to or higher than those we currently see in our term healthy population! Importantly, these countries have breastfeeding cultures, in which breastfeeding in the term healthy population is the norm, with close to 100% initiation.
ADVANTAGES OF HUMAN MILK FOR PREMATURE AND COMPROMISED BABIES
Human milk is a living, changing fluid. It contains over 200 known components, including live lymphocytes, macrophages, neutophils; immunoglobulins, complement, and other host defense factors; lactoferrin; enzymes and hormones such as corticosteroids, erythropoietin, and insulin to name a few, in addition to its nutrients. There are complex interactions between these components, which likely enhance and contribute to their functions. It is ever-changing, from the beginning to the end of a feeding session, throughout the course of the day, and over the course of lactation. It can never be replicated, no matter how much advertising to the contrary is implied. Adding a “new” component to artificial formula that is present in human milk does not in any way guarantee that its function or performance will be identical. Human milk has changed and adapted over the course of human evolution to provide exactly what human infants need. It differs from the milk of other mammalian species, including bovines, which have concomitantly evolved to provide what the young of each of those species require to optimally grow and mature.
Accepting that human milk is the species-specific gold standard in nutrition for human babies, the outcomes associated with its use are then the norm to which other forms of nutrition are compared. That being the case, instead of a discussion of the “benefits of breastfeeding” as has been our habit, it is more accurate to look at the “risks of artificial feeding.” There is a large evidence-based body of literature detailing improved health and developmental outcomes of human milk-fed term babies and their mothers, over artificially fed babies, which have been well reviewed elsewhere (1,2,3,4,42,43,44,45,46). The economic savings to families, health care payers, employers, and society have also been studied. Table 23-2 summarizes these disadvantages of using artificial formulas.
TABLE 23-2 SUMMARY OF THE DISADVANTAGES OF NOT BREASTFEEDING | ||
|---|---|---|
|
There is every reason to assume that these same advantages of human milk to term, healthy babies also apply to preterm and sick neonates. Additionally, there is increasing research-based evidence of both short- and long-term positive effects on prematurity-related conditions, including nutrition, gastrointestinal (GI) function, host defense, neurodevelopment, and physiological well-being.
Nutritional Advantages
Both the AAP (1) and the Canadian Paediatric Society (5) strongly recommend that breast milk is not only the preferred nutrition for healthy term infants, but for all infants, including premature and sick newborns, with rare exceptions (1). For an excellent in-depth examination of this topic please see Chapter 22. The reader is also referred to a recent review article of the use of human milk for premature infants (47).
There are several points that are worth reiterating here. Preterm milk is different than term milk (Table 23-3). Notably, preterm milk has higher concentrations of protein, fatty acids, sodium, and chloride (48,49), which interestingly, are all components required in higher amounts by babies born early. This phenomenon was initially attributed to lower milk volumes produced by mothers of preterm babies, thus causing a concentrating effect on these nutrients.
However, contrary to this theory, other components of preterm milk are present in the same concentrations as in term milk. It has subsequently been shown that preterm milk has similar volumes as term milk, so this is a true occurrence. Some have speculated that this is a maternal adaptation to the delivery of her premature baby, although others suggest that these differences are the end result of the interruption in maturation of the mammary gland during pregnancy. In a recent study looking at total nitrogen, fat, lactose and carbohydrate concentrations, gestational age at birth (GA) was inversely related to carbohydrate concentration; postmenstrual age (PMA; an indicator of autonomous developmental processes not affected by the moment of birth) was not related to milk composition; although postnatal age (PNA) was related to a decrease in total nitrogen and an increase in lactose concentration. This data was interpreted to indicate that PNA strongly influences the development of the composition of very preterm human milk, GA affects carbohydrate content with a negligible effect on the nutritional value of the milk, although PMA has no effect (50).
However, contrary to this theory, other components of preterm milk are present in the same concentrations as in term milk. It has subsequently been shown that preterm milk has similar volumes as term milk, so this is a true occurrence. Some have speculated that this is a maternal adaptation to the delivery of her premature baby, although others suggest that these differences are the end result of the interruption in maturation of the mammary gland during pregnancy. In a recent study looking at total nitrogen, fat, lactose and carbohydrate concentrations, gestational age at birth (GA) was inversely related to carbohydrate concentration; postmenstrual age (PMA; an indicator of autonomous developmental processes not affected by the moment of birth) was not related to milk composition; although postnatal age (PNA) was related to a decrease in total nitrogen and an increase in lactose concentration. This data was interpreted to indicate that PNA strongly influences the development of the composition of very preterm human milk, GA affects carbohydrate content with a negligible effect on the nutritional value of the milk, although PMA has no effect (50).
TABLE 23-3 COMPARISON OF PRETERM TO TERM HUMAN MILK | ||||||
|---|---|---|---|---|---|---|
| ||||||
The higher concentration of nutrients in preterm human milk all decrease to approximately term milk levels over the course of the first postnatal month, regardless of the gestational age of the baby at delivery, although the premature baby’s increased needs continue until approximately term corrected gestational age. With the advantage the earlier higher concentrations, particularly of protein and electrolytes, that premature milk afforded no longer being present, we are often required to fortify human milk for the smallest babies. Babies less than 1,500 grams have been shown to require fortification with more calories, protein, calcium, phosphorus, sodium chloride and some vitamins to preclude poor growth rates, hyponatremia, hypochloremia, and osteopenia (47). Larger, more mature babies thrive on mother’s milk alone. Because human milk content differs not only over the course of lactation, but during the course of a feed or milk expression session, at different times of the day, and for those babies requiring feeding by alternative methods, by the method used, it is critical to monitor these very low birth weight babies for growth rates, serum sodium levels, and bone mineralization status (see Chapter 22).
Protein
Human milk protein is 80% whey, as opposed to bovine milk protein, which is 80% casein. The whey in human milk, α-lactalbumin, is much more easily digested than bovine whey, which is β-lactalbumin, an important factor to consider for premature babies with immature gut function. Additionally, human milk protein also includes nucleotides, secretory immunoglobin A (sIgA) and other immunoglobulins, and an enzyme, lysozyme, all of which are thought to aid in host defense; growth factors that stimulate gut growth and maturation; a variety of hormones; and enzymes (e.g., mammary amylase, lipases) that enhance the immature intestinal tract’s ability to digest nutrients. The amino acid taurine, which serves many functions in the newborn including bile acid conjugation, osmoregulation, neurotransmission, and as an antioxidant and a growth factor, is present in high concentrations in human milk and is almost absent in bovine milk. Hence, it is added to artificial feedings. Unlike bovine milk, human milk is also low in phenylalanine and tryrosine, which the premature and newborn infant are poorly equipped to metabolize. For these reasons among others, human milk protein composition is well-adapted to the needs of premature babies (see Chapter 22) (51).
Lipids
Human milk lipids have sparked the greatest interest in milk components recently, with the increasing body of literature on the positive neuro-developmental effects of the long-chain polyunsaturated fatty acids (LC-PUFA’s), in particular docosahexaenoic acid (DHA) and arachidonic acid (AA). They are found in phospholipids in the brain, retina and red blood cell membranes. These LC-PUFA’s are not readily synthesized by preterm babies, and are normally delivered via the placenta. They occur naturally in human milk, but are not found in bovine milk. For premature babies, they must be delivered via an external source, in this case easily by human milk. Because of studies that show improved neuro-developmental outcome and visual function in breastfed preterm babies and in formula-fed preterm babies supplemented with exogenous sources of LC-PUFA’s, DHA and AA have now been added commercially to most term and preterm infant formulas. Of concern is that these additives are of plant origin and are structurally different than human LC-PUFA’s (see Neurodevelopmental Advantages).
Human milk lipids are an easily digested source of energy, in part as a result of their composition and in part as a result of their packaging with lipases in the milk, providing approximately 50% of the calories.
Additionally they provide cholesterol, which is an essential component of membranes. Human milk-fed babies show significantly higher plasma cholesterol levels than formula-fed babies (52). Although one might then expect them to have higher cholesterol levels than formula-fed babies as adults, the opposite has been found to be true (53). Additionally, coronary artery disease has been shown to be less frequent in persons up to 20 years of age who were initially breastfed (54). It has been postulated that this early exogenous exposure to cholesterol, a necessary nutrient, keeps endogenous cholesterol production down-regulated, thus resulting in lower cholesterol levels in adult life. The mechanisms remain to be elucidated.
Additionally they provide cholesterol, which is an essential component of membranes. Human milk-fed babies show significantly higher plasma cholesterol levels than formula-fed babies (52). Although one might then expect them to have higher cholesterol levels than formula-fed babies as adults, the opposite has been found to be true (53). Additionally, coronary artery disease has been shown to be less frequent in persons up to 20 years of age who were initially breastfed (54). It has been postulated that this early exogenous exposure to cholesterol, a necessary nutrient, keeps endogenous cholesterol production down-regulated, thus resulting in lower cholesterol levels in adult life. The mechanisms remain to be elucidated.
Carbohydrates
The disaccharide lactose is the predominant carbohydrate in human milk. It is a ready source of energy, and is broken down by the enzyme lactase, located in the brush border of the intestinal mucosa, to galactose and glucose, necessary for energy supply to the rapidly growing brain. Lactase activity is low in premature babies, but is readily inducible by exposure to lactose, enabling them to absorb more than 90% from human milk. The remaining unabsorbed lactose contributes to softer stool consistency, and colonization of the gut by nonpathogenic fecal flora. It also enhances calcium absorption, critical to preventing nutritional rickets in prematures. Oligosaccharides, present in human milk as well, act in host defense by preventing bacterial attachment to intestinal mucosa, thus serving a protective role for the relatively immunocompromised preterm infant.
Energy
Several previous studies have suggested that human milk-fed term (55,56) and preterm (57,58) infants have lower sleeping energy expenditure compared with formula-fed infants. A recent randomized, cross-over study of gavage-fed preterm babies showed significantly lower energy expenditure in the human milk fed babies at prefeeding, during feeding, and postfeeding measurements (59). With the vast difference in composition of the nutrients and other factors between human and artificial milk, it is not possible to say what causes this difference, but it is an intriguing difference that remains to be investigated.
Gastrointestinal Advantages
In addition to the species specificity and superior digestibility of the nutrients in human milk for premature babies, human milk also favorably affects the function and maturation of the GI tract. It has been shown in vivo to decrease intestinal permeability in preterm infants when compared to preterm formula (60). Several studies have found that human milk promotes more rapid gastric emptying than artificial formula (61,62), with one revealing that on average, human milk emptied twice as fast as formula (62). This has implications for clinical practice. Delayed gastric emptying, which generally presents clinically as measured gastric residuals or vomiting, prevents advancement of enteral nutrition. Babies who cannot reach full enteral feeds require longer periods of parenteral nutrition, with the concomitant risks inherent in both prolonged intravenous catheter usage (e.g., infection, thrombosis, chemical infiltrates) and prolonged intravenous nutrition (e.g., mineral or electrolyte imbalance, hepatic damage). Any of these can impact length of stay, which in turn has economic and social/family implications, all of which are important to consider in the therapeutic plan in our NICUs today. In fact, other studies suggesting that human milk is better tolerated by the GI tract of premature babies than formula have looked at the marker “time to full feeds.” Several have shown that infants fed human milk achieve full enteral feeds significantly faster than those fed artificial formulas (63,64). This would also be expected to have a positive impact on length of stay.
Another related finding is the induction of lactase activity by feedings. Lactase, the enzyme responsible for the digestion of lactose, is present in the fetal intestine early in gestation, but the greatest increase occurs during the third trimester. Hence, premature babies are lactase-deficient at birth. In one study, lactase activity was induced in preterm babies (26-30 weeks gestation) by the initiation of enteral feeds. Of greatest significance, the highest levels of enzyme activity were seen with the introduction of “early” feeds (4 days old) as opposed to “standard” feeds (15 days old) and in human milk-fed vs. formula-fed babies (65). There was also an inverse correlation between lactase activity at 28 days, and the time to achieve full enteral feeds. It appears that the level of lactase activity may be a marker of intestinal maturity, with human milk use being directly related to the progression of that maturity.
There are a large number of “bioactive components” in human milk that are not present in formulas. They variously provide antiinflammatory effects or protection from infectious agents; or are hormones and growth factors that influence development; or are immune function modulators (66). This is an active area of research with many questions remaining to be answered. At least a few of these factors have activity suggesting they may be involved in GI maturation, growth and motility (67). Epidermal growth factor (EGF) is a major growth-promoting cytokine that stimulates proliferation of intestinal mucosa and epithelium and strengthens the mucosal barrier to antigens (68,69). In an animal model, EGF isolated from human milk has been shown to facilitate gut healing after induced injury (69). Other factors identified in human milk, known as human growth factors I, II and III and insulin-like growth factor, have been shown to have growth-promoting functions, including stimulation of deoxyribonucleic acid (DNA) synthesis and cellular proliferation. In vivo studies in animal species have shown remarkable increases in the mass of intestinal mucosa after feedings with colostrum, which contains these factors, but not after feedings with artificial milk (70,71). It is intriguing to postulate that
factors such as these in human milk may be important to the maturation and development of the premature GI tract, as well as being important to intestinal repair after damage as a result of disease processes such as necrotizing enterocolitis.
factors such as these in human milk may be important to the maturation and development of the premature GI tract, as well as being important to intestinal repair after damage as a result of disease processes such as necrotizing enterocolitis.
Host Defense Advantages
One of the single most extraordinary advantages of the use of human milk over formula in the preterm and NICU patient is the effect on host defense and infections. This reason alone is enough to make the use of human milk the gold standard in this population. The myriad of hormones, factors, cytokines, proteins, enzymes, nucleotides, antioxidants, immunoglobulins, and live, functioning leukocytes contained within human milk (72), their interactions and the milieu in which they effect action, is not and cannot ever be duplicated in an artificial milk. And our knowledge is just beginning to scratch the surface.
One can think of human milk as not only the perfect form of nutrition, but also as “baby’s first immunization.” One type of immunization is the passive transfer of antibody. Examples include the use of intravenous immune globulin, tetanus immune globulin, rabies immune globulin, or respiratory syncytial immune globulin. The immunoglobulins represented in a mother’s milk are a history of many of the infectious agents she has been exposed to, and developed antibody to, during her lifetime. By transferring these antibodies to her baby through her milk, she is then in effect “immunizing” her baby against those organisms. This process is a continuum from the one begun in utero by the passage of maternal antibody across the placenta. Taking this one step further is the concept of the enteromammary immune system, first proposed in 1979 by Kleinman and Walker (73) (Fig. 23-1). In this schema, antigen presented to the maternal mouth and gut is brought into proximity of lymphoid follicles in the maternal GI tract. The antigen’s presence commits maternal lymphoblasts to specific IgA production against that antigen. These lymphoblasts migrate via the mesenteric nodes and the thoracic duct into the systemic circulation, in which they find their way into the active mammary tissue. There these cells manufacture sIgA, which is secreted into the milk. When the infant ingests the milk, the immunoglobulin present functions in the infant’s gut as protection against that specific pathogen. Most sIgA is not absorbed in the infant’s intestine, but rather plays an active role in mucosal defense. Although intact immunoglobulin has been found in infant urine, signifying some systemic absorption, most survives intact in the GI tract, and is excreted into the feces. The environment of the neonatal intensive care unit is full of potentially pathogenic organisms. What makes this concept of the enteromammary immune system even more appealing is that during skin to skin (kangaroo) care, a neonate is held by the mother at her chest, and is touched, kissed, and caressed. The mother exposes herself to any potential pathogens to which her baby has also been in contact. Through the enteromammary immune system, she makes antibody to those particular organisms, and then in subsequent feedings, she passes that antibody to her baby, to help protect him/her. Imagine—individually prepared immunizations to help protect each different baby in the NICU!
Similarly to our previous discussion of concentrations of specific nutrients, many of these immune modulators are in higher concentration in preterm milk than term milk, helping to compensate for the premature baby’s immature immune function (Table 23-4). When major factors were quantified and compared between colostrum of mothers delivering both prematurely (28-36 weeks) and at term (38-40 weeks), mean concentrations of IgA, lysozyme, lactoferrin, and absolute counts of total cells, macrophages, lymphocytes, and neutrophils were found to be significantly higher in the preterm colostrum (74). The degree of prematurity did not influence the antiinfective levels in the colostrum. However, the total cells and macrophages were significantly higher in the colostrum of mothers delivering at 28-32 weeks of gestation compared to 33-36 weeks gestation (p < 0.05) (74). These differences make the use of early colostrum and human milk critical in the care of premature and sick babies, both as prevention and also possibly in treatment of infectious disease.
Another likely factor in improved immune defense with the use of human milk is the affect on fecal flora. The normal flora of the intestines of a breastfed infant is predominantly the gram positive bacteria Lactobacillus bifidus. Non-human milk-fed babies are colonized with many more types of bacteria, most of which are the potentially pathogenic gram negative bacteria. With establishment of Lactobacillus as the predominant bacteria inhabiting the premature infant’s GI tract, the likelihood of a serious or life-threatening gram negative infection would be expected to decrease.
It has long been known that breastfed babies are at decreased risk of a number of infectious diseases—respiratory infections, otitis media, gastroenteritis, and diarrhea—and are at decreased risk of mortality. These protections accrue in a dose-dependent fashion—i.e., the more breast milk a baby receives, statistically the better protected they are. Although much of the earlier work was done in Third-World countries, and commonly held beliefs are that breastfeeding does not make a difference in industrialized nations like the United States, there are now many studies that show significant impact in these populations as well (75,76,77,78). There has even been a study looking at breast-feeding and the risk of postneonatal deaths in the United States which shows promoting breastfeeding has the potential to save or delay about 720 postneonatal deaths each year (79)! It is probably fair to assume that babies admitted to NICUs, once attaining term corrected gestational age and discharged to home, will accrue similar advantages from breast milk/breastfeeding as their healthier term counterparts in these studies. But even more importantly for our population, an increasing body of research has been done looking at the effects of a diet of
human milk compared to preterm formula in premature and low birth weight babies, with respect to clinical infections. The data is clear—premature babies fed human milk are at significantly less risk of serious diseases, including necrotizing enterocolitis, urinary tract infections, sepsis, and meningitis.
human milk compared to preterm formula in premature and low birth weight babies, with respect to clinical infections. The data is clear—premature babies fed human milk are at significantly less risk of serious diseases, including necrotizing enterocolitis, urinary tract infections, sepsis, and meningitis.
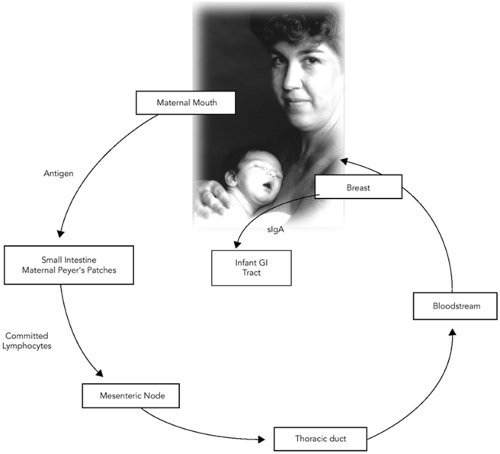 Figure 23-1 The Enteromammary Immune System. Adapted from Kleinman RE, Walker, WA. The enteromammary immune system: an important new concept in breast milk host defense. Dig Dis Sci 1979;24:880. |
As early as 1971, a report from Sweden showed a protective effect of breastfeeding against sepsis in the newborn (80). Then in 1980, Narayanan and her group in India reported that even partial use of human milk (81), and subsequently exclusive use (82) could significantly decrease the incidence of infection in a premature and low birth weight population. In the first study, infants fed human milk supplemented with formula had a 6% incidence of sepsis, compared to a 21% incidence in those fed formula alone (81). In the second, in 62 infants studied, no sepsis occurred in those infants receiving human milk exclusively, although six episodes occurred in those fed formula (82). A further study by this group looked at the dose-response effect of human milk in the prevention of infection (83). Infections noted were sepsis, diarrhea, pneumonia, meningitis, conjunctivitis, pyoderma, thrush, and upper respiratory infections. The strongest effect was noted in those babies fed exclusive human milk, followed by partial feedings of human milk. In a United States study, a decrease in blood culture proven sepsis was seen in those infants fed their own mothers’ milk compared to those fed formula (27% vs. 58%, p < 0.05) (63) More recently in another U.S. neonatal unit, El-Mohandes and associates showed a significantly decreased incidence of sepsis, in each of three time periods through the first 38 days of life, for infants admitted to the NICU who received human milk vs. formula (odds ratio for sepsis in human milk-fed infants was 0.4, 95% confidence limits, 0.15 to 0.95, p =0.04) (84).
TABLE 23-4 COMPARISON OF ANTIINFECTIVE PROPERTIES OF PRETERM AND TERM MOTHER’S COLOSTRUM | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||
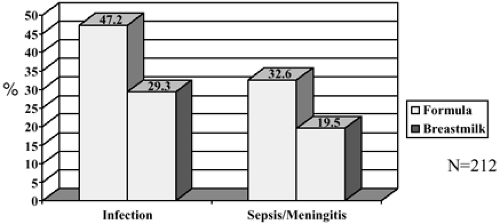
Hylander and colleagues published an elegant study in 1998 in which they looked at 212 VLBW infants born at Georgetown University Medical Center (85). They not only looked at incidence of infection among these babies in relationship to their type of feeding, but they also controlled for confounding factors. Infection was documented as clinical signs of sepsis along with positive cultures for pathogenic organisms from one or more of the following sites: blood, spinal fluid, urine, stool, pleural fluid, nasopharyngeal, intravascular catheter, umbilicus, eye, or surgical wound. Additionally, pneumonia (by chest radiograph) and necrotizing enterocolitis (Bell’s classification) were included. Human milk feeding was defined as receiving any human milk, with supplemental formula feedings when milk was not available. Formula-fed babies received only formula. They found that the incidence of infection (human milk 29.3% vs. formula 47.2%) and sepsis/ meningitis (human milk 19.5% vs. formula 32.6%) differed significantly by type of feeding (Fig. 23-2). Human milk was independently correlated with a reduced odds ratio of infection (OR = 0.43, 95% CI, 0.23-0.81), controlling for gestational age, 5-minute APGAR score, days on mechanical ventilation, and days without enteral feeds. Human milk feeding was also independently correlated with a reduced odds ratio of sepsis/meningitis (OR = 0.47, 95% CI 0.23-0.95), controlling for gestational age, mechanical ventilation days, and days without enteral feeds. And remember, the human milk-fed babies were supplemented with formula, so if they had been exclusively breastfed, one wonders if the difference would have been even more remarkable?
In 1999, Schanler and his colleagues showed that in a group of premature infants between 26 to 30 weeks gestation, those fed predominantly fortified human milk were discharged earlier (73 ±19 vs. 88± 47 days) and had lower incidence of necrotizing enterocolitis (NEC) and late-onset sepsis than infants fed preterm formula (86). This data on NEC confirms a previous study by Lucas and Cole (87), who showed that in a cohort of 926 infants with birthweights below 1850 grams, formula fed babies were six to ten times more likely to develop NEC than those fed human milk exclusively; and three times more likely than babies who received a combination of human milk and formula supplements. Once again, this data shows a dose-response effect of human milk. While reviewing the data on NEC it is also important to point out that in addition to these significant clinical decreases in incidence of NEC, there are also concomitant significant savings in economic costs. This is an enormous issue to us as practitioners, to the health care system as a whole, and certainly to the individual families we care for. A reduction in the cases of NEC would engender significant savings in medical charges, and length of stay (LOS). Bisquera and associates (88) showed that infants with surgical NEC exceeded LOS by 60 days over matched controls, and medical NEC by 22 days over
controls. Based on LOS, the estimated total hospital charges per baby for surgical NEC averaged $186,200 more and medical NEC $73,700 more than controls. This translated into yearly additional hospital charges at their institution for NEC of $6.5 million, or $216,666 per survivor! When parents’ time and wages and prevention of premature deaths are also considered, the total savings in the United States alone are more than $3 billion if only 75% of preterm infants received human milk feedings and their incidence of NEC decreased from 7% to 1% (89)! The significance of these statistics cannot be ignored.
controls. Based on LOS, the estimated total hospital charges per baby for surgical NEC averaged $186,200 more and medical NEC $73,700 more than controls. This translated into yearly additional hospital charges at their institution for NEC of $6.5 million, or $216,666 per survivor! When parents’ time and wages and prevention of premature deaths are also considered, the total savings in the United States alone are more than $3 billion if only 75% of preterm infants received human milk feedings and their incidence of NEC decreased from 7% to 1% (89)! The significance of these statistics cannot be ignored.
Just as the etiology of NEC is not completely understood, and is certainly multi-factorial, so the mechanism underlying the protection against NEC afforded by breast milk is also not completely understood, and most likely multi-factorial. One study showed a decreased prevalence of NEC in premature infants who were given enteral doses of a serum-derived IgA-IgG product, with IgA predominance, similarly to that found in human milk (90). This leads one to postulate that the immunoglobulins, in particular IgA, present in human milk have a role to play in NEC prevention. It is also known that platelet-activating factor (PAF) is one of the inflammatory mediators that is known to be higher in babies with NEC, and that in experimental models, it causes bowel necrosis similar to that seen in NEC, when injected intravenously. PAF is rapidly broken down by PAF acetylhydrolase, which has been shown to be not only present in human milk, but to be approximately 5-fold higher in preterm than in term milk (91). This may yet be another mechanism by which breast milk is protective. Additionally we have previously discussed the predominance of nonpathogenic fecal flora in breastfed infants. Could this not be another protective mechanism? There are certainly many other biologically active substances in human milk that may ultimately be found to have a role in the prevention of NEC.
It is unclear how much human milk is needed to see these effects. In the previously discussed Hylander study (85), the human milk-fed group also received supplements of formula and still showed significant benefit. Narayanan (83) showed a dose-response in that exclusive human milk was more protective than partial, which was still more protective than no human milk. And Lucas and Cole showed that in NEC, partial human milk feeding was protective, but less so than exclusive human milk feedings (87). Furman and colleagues, looking at 119 VLBW babies, identified a daily threshold amount of at least 50 ml/kg/day of maternal milk through week 4 of life as needed to decrease rates of sepsis in this group (92). These data are convincing that by providing human milk, we do make a difference in these serious morbidities in even the tiniest of our patients. And it certainly appears that there is a dose-response relationship. That being the case, with all the potential morbidities that our patients face, and the costs both economically and emotionally for these families and society, it is without a doubt worth our efforts to make human milk the gold standard in neonatal intensive care for these reasons alone. It has also been shown that upper respiratory symptoms are reduced for those low birth weight babies through seven months corrected age who continue to receive human milk after discharge from the NICU (93). Although not significant, a trend appears to exist for otitis media, bronchiolitis, and gastroenteritis as well. More studies with larger cohorts are needed to confirm these data.
Neurodevelopmental Advantages
Much press has been given to recent work that shows improved cognitive development in babies who receive human milk. In 1988 Morley and associates showed an 8-point cognitive advantage using the Bayley Scales of Infant Development for 771 infants with birth weights less than 1850 grams (94). After controlling for demographic and perinatal factors, a 4.3-point advantage still remained. When this cohort was followed up at 7.5 to 8 years of age, infants who had received human milk by tube (rather than breastfeeding) continued to show an 8.3-point IQ advantage (over half a standard deviation) even after adjustments were made for difference in mother’s education and social class (95). They also showed a dose response relationship between the proportion of human milk in the diet and subsequent IQ. In a second randomized prospective study, these same researchers compared premature infants who received mature donor human milk with those who received premature formula as their early enteral nutrition. These diets were compared as sole enteral feeds or as supplemental feeding to their own mother’s expressed milk. No differences in outcome were seen at 18 months between the two diet groups, despite the low nutrient content of mature donor milk in relation to preterm formula. Additionally, when they compared the infants from this study fed solely on mature donor milk with infants from a previous study fed solely standard term formula, the infants fed the mature donor milk had higher developmental scores, again supporting the positive role of human milk on cognitive development (96). Bier and colleagues have also looked at the effects of human milk on both cognitive and motor development. They found that in a cohort of 39 premature infants, those who received human milk had significantly higher motor scores at 3 and 12 months corrected ages than those fed formula. Additionally, when adjusting for oxygen requirement and maternal vocabulary scores, human milk-fed babies continued to show an advantage in both cognitive and motor scores at 12 months. There was also an association between the amount of human milk intake while in the special care nursery (dose response relationship!), and cognitive development at both 7 and 12 months corrected ages (97).
There has been one meta-analysis of controlled studies looking at the question of human milk and cognitive development (98). It demonstrated a 3.16-point higher score for cognitive development in human milk-fed babies compared with formula after adjustment for significant covariates. This difference was observed as early as 6
months and was sustained through 15 years of age, the last time of reliable measurement. Longer duration of breast-feeding was accompanied by greater differences in cognitive development (dose related response). Whereas normal weight infants showed a 2.66-point difference in IQ scores between breastfed and formula fed groups, the difference was even more remarkable, a 5.18-point difference, in low-birth-weight infants. The results of this meta-analysis suggest that not only does human milk contribute to higher neurodevelopment, but that the effect is even more striking in a much more high-risk population, the low-birth-weight infant.
months and was sustained through 15 years of age, the last time of reliable measurement. Longer duration of breast-feeding was accompanied by greater differences in cognitive development (dose related response). Whereas normal weight infants showed a 2.66-point difference in IQ scores between breastfed and formula fed groups, the difference was even more remarkable, a 5.18-point difference, in low-birth-weight infants. The results of this meta-analysis suggest that not only does human milk contribute to higher neurodevelopment, but that the effect is even more striking in a much more high-risk population, the low-birth-weight infant.
Postulated to be responsible for at least a portion of these neurodevelopmental advantages of human milk is the presence of long-chain polyunsaturated fatty acids (LC-PUFAs), which until recently, were not present in formulas (Please see Chapter 22 for a more in-depth review). Docosahexaenoic acid (DHA), normally accounts for greater than one-third of the total fatty acids of the gray matter of the brain and the retina of the eye (99). Most of the prenatal accumulation of DHA in these tissues occurs in the third trimester—thus by definition, premature infants are deficient compared to their term counterparts. Animal studies have shown that deficiency of DHA in neural tissues during development leads to behavioral and retinal changes.
Other examples of the effects of human milk on neurological maturation have also been observed. Premature infants receiving human milk have been shown to have faster brainstem maturation than those on formula (100). Visual acuity and the development of retinopathy of prematurity have also been studied. A number of studies were performed before the routine addition of the LC-PUFAs to preterm formulas. In one, the researchers showed improved visual acuity of preterm infants up to four months of age in supplemented vs. nonsupplemented formulas (99). In another, improved retinal function was present in LC-PUFA sufficient VLBW neonates fed human milk or supplemented formula as compared to an unsupplemented formula group with lower LC-PUFA cell composition (101). Better visual evoked potentials and acuity in both preterm and term infants at 57 weeks postconception has been seen in those fed human milk as opposed to those fed formula (102). Additionally, another interesting study in which healthy term infants who breastfed to 4 or 6 months, and then were weaned, were randomly assigned to commercial formulas with or without DHA and arachidonic acid (ARA) supplements. At 1 year of age, the level of DHA as measured in the red blood cells was reduced by 50% from weaning level in the unsupplemented group, although there was an increase of 24% in the supplemented group (103). The conclusions drawn from this study were that the critical period during which a dietary supply of DHA and ARA can contribute to optimizing visual development in term infants extends through the first year of life. This supports the AAP recommendation that breastfeeding continue through the first year of life (1), and begs consideration then of the length of time breastfeeding should be encouraged and supported for the baby born prematurely.
Physiological Advantages
Breastfeeding is widely assumed to be more stressful than bottle feeding for premature infants, an assumption that has led in many intensive care units to the introduction of bottle feedings as the first oral feeds, and postponing attempts at breastfeeding until babies “can prove themselves with the bottle.” There are also often concerns for the small premature baby’s ability to maintain temperature while breastfeeding, leading to “rules” restricting initiating breastfeeding until a certain weight is achieved. Additionally, there is the widely held belief that the “suck-swallow-breathe” mechanism is not mature until approximately 34 weeks gestation. Because of the fear that they will choke, desaturate and aspirate, this leads to more “rules” concerning not initiating oral feeds, including breastfeeding, until at least 34 weeks corrected gestational age. It is important to point out that there is no scientific evidence to back any of these statements. On the contrary, the exact opposite is the case as we will discuss. It is also important to understand that these policies, in addition to being unsupported scientifically, are also harmful in that they (a) preclude premature babies and their mothers from early breastfeeding experiences; (b) allow babies whose mothers want to breastfeed to learn to suck from a bottle, which then in many cases makes it hard for them to transfer to the breast at a latter time, as the sucking mechanisms are different; and (c) introduces breastfeeding so late in the hospital stay, that in addition to struggling to overcome what they have learned with an artificial nipple, the mother and baby are often discharged before they have had time to learn together and develop the confidence and skills necessary to allow successful breastfeeding in this population. It is important to re-emphasize our medical dictum in this circumstance: Primum non nocere.
Data does exist that enables us to develop breastfeeding policies that are physiologic, safe and supportive of breast-feeding. As early as the 1980s, Meier was publishing data concerning physiologic stability at breast compared to bottle-feeding. She was able to show that in babies less than 1,500 grams at the time of first feeding, different sucking mechanisms were employed for breast and bottle, with better coordination of suck-swallow-breathe during breastfeeding, particularly in the smaller less mature babies. Concomitantly, there are markedly different patterns of transcutaneous oxygen pressure (tcPO2) for the two methods of feeding. TcPO2 patterns suggest less ventilatory interruption during breastfeeding than during bottle-feeding with greater declines in tcPO2 during bottle-feeding than during breast-feeding over the course of a feeding session. Additionally, babies became significantly warmer during breastfeeding than during bottle-feeding (104,105,106). Similar studies done by Bier and colleagues in first VLBW (107), and then ELBW (108) babies showed they could tolerate beginning breast- and bottle feedings at the same postnatal age; that they were
less likely to have oxygen desaturations to less than 90% during breastfeeding; and that they had lower intakes during breastfeeding. Given that there is no data supporting the safety of initiating bottles as first feeds, and that there is data to show that during breastfeeding premature babies are more physiologically stable, it would then seem reasonable, safe and scientifically sound to allow mothers to put their babies to nuzzle at breast and to begin early steps toward breastfeeding, once they are physiologically ready!
less likely to have oxygen desaturations to less than 90% during breastfeeding; and that they had lower intakes during breastfeeding. Given that there is no data supporting the safety of initiating bottles as first feeds, and that there is data to show that during breastfeeding premature babies are more physiologically stable, it would then seem reasonable, safe and scientifically sound to allow mothers to put their babies to nuzzle at breast and to begin early steps toward breastfeeding, once they are physiologically ready!
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree