Board Simulation: Acid-Base and Complex Fluid and Electrolyte Problems
Robert J. Cunningham III
In this chapter, a board simulation format is utilized to review acid-base physiology and transmit an approach that will allow you to tackle more complex fluid problems. A number of cases are presented with relevant questions. The correct answers and the logic used to arrive at each answer appear following each question.
QUESTIONS
Case 1
A 6-year-old boy is being evaluated for short stature. His physical examination findings are normal, except that his height is below the fifth percentile. His height was in the fifth percentile when he was 3 years old and has slowly fallen off the curve to just below the fifth percentile. His weight is proportional to his height.
His urinalysis is normal, with a urinary pH of 6.5. No protein, blood, or white blood cells are noted. His electrolyte values are as follows:
Na = 141 mEq/L
K = 3.9 mEq/L
Cl = 110 mEq/L
HCO3 = 19 mEq/L
1. The data are most consistent with which of the following?
a. Chronic renal failure
b. Diabetic ketoacidosis
c. Urea cycle defect with acidosis
d. Renal tubular acidosis (RTA)
View Answer
Answer
The answer is d. When you are confronted with a patient with evidence of acidosis, the first step is to calculate the anion gap, as shown:
Na − (Cl + HCO3) = 141 − (110 + 19) = 12
A normal value for the anion gap is from 8 to 16. Acidosis with a normal anion gap can be caused by:
Renal loss of bicarbonate
RTA
Carbonic anhydrase inhibitors
Posthypocapnia
Gastrointestinal loss of bicarbonate
Diarrheal stool
Ileostomy drainage, digestive fistulae
Ileal conduits
Administration of cation exchange resins
Administration of acid
Arginine chloride, hydrochloric acid
Parenteral nutrition
Dilution acidosis
The origin of the anion gap is that under normal circumstances, the Na+ ions are balanced by the sum of the Cl and HCO3 ions, in addition to other anions not accounted for in the equation. The anion gap of 8 to 16 accounts for these ions, which include PO4, albumin, and other protein molecules that carry a negative charge. There is no net charge because the numbers of positively and negatively charged molecules in the circulation are equal. A normal value for the anion gap is 8 to 16, so that the gap in this example is well within the normal range. All causes of a normal anion gap acidosis represent situations of either a direct loss of bicarbonate or an addition of acid to the body fluids. In nearly all cases, you are directed to the kidney or gastrointestinal tract, the two organs with the capacity to eliminate bicarbonate.
Situations of active addition of H+ to the blood are special. The one most often encountered in practice is the patient who is dependent on total parenteral nutrition. In this scenario, no additional anion is present in the circulation. The mechanism for the development of acidosis is loss of bicarbonate; and with each loss of a bicarbonate ion, a cation (e.g., Na+) is lost simultaneously. In this way, electrical neutrality is maintained, and a normal anion gap is preserved.
Situations of active addition of H+ to the blood are special. The one most often encountered in practice is the patient who is dependent on total parenteral nutrition. In this scenario, no additional anion is present in the circulation. The mechanism for the development of acidosis is loss of bicarbonate; and with each loss of a bicarbonate ion, a cation (e.g., Na+) is lost simultaneously. In this way, electrical neutrality is maintained, and a normal anion gap is preserved.
In the earlier example, assume that you are considering the diagnosis of RTA and that you obtained a first morning urine sample along with a blood gas. The results are shown in the subsequent text:
Urinalysis | Blood Gas |
|---|---|
pH = 6.0 | pH = 7.34 |
Specific gravity = 1.025 | HCO3 = 18 mEq/L |
Glucose, negative | PCO2 = 32 mm Hg |
Protein, negative | |
Hemoglobin, negative |
2. These results effectively:
a. Confirm a diagnosis of type I (distal) RTA
b. Confirm a diagnosis of type II (proximal) RTA
c. Exclude RTA as a cause of acidosis
d. Establish acidemia without identifying a specific defect
View Answer
Answer
The answer is d. You have now established that the patient has acidemia; the bicarbonate is low, the pH is low, and a compensatory low PCO2 is present. How do you know that this is a metabolic acidosis and not a compensatory response to a respiratory alkalosis? The answer is that the pH is <7.40, and overcompensation never occurs.
After the administration of ammonium chloride, an acid, the following results were obtained:
Urinalysis | Blood Gas |
|---|---|
pH = 6.0 | pH = 7.29 |
Specific gravity = 1.014 | HCO3 = 14 mEq/L |
PCO2 = 32 mm Hg |
3. The diagnosis is consistent with:
a. Type I distal RTA
b. Type II proximal RTA
View Answer
Answer
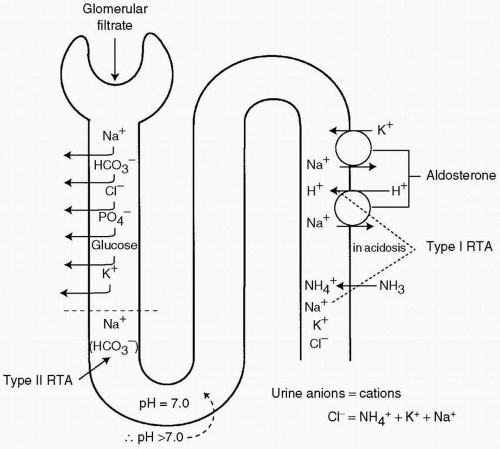
The answer is a. This patient has received an acid load, and even in the face of severe acidosis, the urine has a pH of 6.0. This finding indicates a distal tubular defect and an inability to excrete acid. The normal response to the acid load would be to excrete enough H+ to drive the urine pH to a value of 5.5 or lower. Hence, a pH of 6.0 indicates that the distal tubule function is inadequate. If the patient had a type II (proximal) RTA, with a bicarbonate level of 15 mEq/L, the proximal tubule would reabsorb all the bicarbonate presented to it. As shown in Figure 14.1, the pH of the fluid entering the distal tubule would be 7.0, and with the addition of acid by distal tubular cells, the pH would fall to at least 5.5, if not lower.
4. Further history that would increase your suspicion of RTA would include:
a. Father with a history of polycystic disease
b. Mother with a history of renal stones
c. Father with a history of anemia
d. Sibling who is also in the fifth percentile for height
View Answer
Answer
The answer is b. In an individual with a chronic inability to excrete an acid load, H+ is buffered in the bone. For every 2 H+ ion that is buffered in the bone, one Ca2+ ion is released and must be excreted. As a result, hypercalciuria develops and predisposes the patient to the formation of renal stones. When renal stones develop in an adult, RTA must be considered as a predisposing cause.
5. If this child is begun on therapy with sodium bicarbonate or sodium citrate, what is the major side effect to be avoided?
a. Hypokalemia
b. Metabolic alkalosis
c. Volume overload
d. Hypernatremia
View Answer
Answer
The answer is a. Hypokalemia develops in patients with RTA and is exacerbated by treatment with base solutions containing sodium but not potassium. The reason is that the patient is acidotic, and the aldosterone level is elevated in an attempt to increase H+ excretion. K+ is excreted by the same mechanism; K+ ions are excreted in the distal tubule in exchange for Na+ ions. When a patient is treated with sodium solutions, the result is decreased sodium reabsorption in the proximal tubule and enhanced delivery of Na+ to the distal tubule. With a high aldosterone level and an increase in the amount of substrate available for exchange, potassium excretion is enhanced and hypokalemia results. This process is illustrated in Figure 14.2.
Case 2
A 6-year-old boy is brought to the emergency department in respiratory distress. His breathing rate is 44/minute, and his respirations are deep. The physical examination shows that he is moving air extremely well and has no rales, wheezes, or rhonchi. Blood pressure is 130/70 mm Hg, pulse 90/minute, temperature 37.4°C (99.32°F), and weight 25 kg. He had a viral illness 7 to 10 days ago with diarrhea but seemed to be doing well with a good appetite. Initial blood gas and electrolytes determination shows:
pH = 7.25
PCO2 = 20 mm Hg
Na = 128 mEq/L
K = 5.1mEq/L
HCO3 = 5 mEq/L
Cl = 98 mEq/L
6. This clinical presentation is consistent with all of the following except:
a. Acute renal insufficiency (uremia)
b. Salicylate intoxication
c. Diarrhea
d. Diabetic ketoacidosis
View Answer




Answer
The answer is c. Diarrhea would result in a nongap acidosis. The gap here is 128 − (98 + 5), or 25. This is an elevated anion gap and therefore is not consistent with a gastrointestinal loss of bicarbonate. In cases of gap acidosis, the mnemonic MUDPILES is helpful. The causes of gap acidosis are outlined in Table 14.1.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree