Chapter 97 Blood Disorders
97.1 Anemia in the Newborn Infant
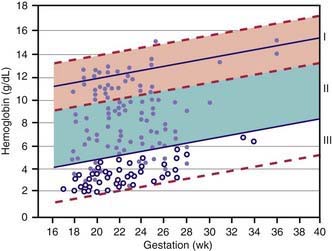
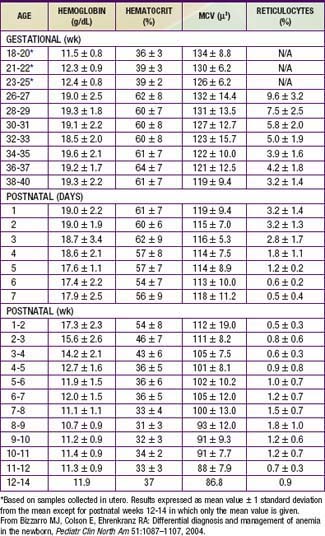
Hemoglobin increases with advancing gestational age: at term, cord blood hemoglobin is 16.8 g/dL (14-20 g/dL); hemoglobin levels in very low birthweight (VLBW) infants are 1-2 g/dL below those in term infants (Fig. 97-1). A hemoglobin value less than the normal range of hemoglobin for birthweight and postnatal age is defined as anemia (Table 97-1). A “physiologic” decrease in hemoglobin content is noticed at 8-12 wk in term infants (hemoglobin, 11 g/dL) and at about 6 wk in premature infants (7-10 g/dL).
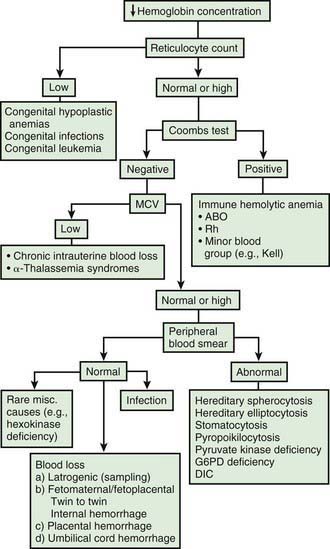
Infants born by cesarean section may have a lower hematocrit (Hct) than those born vaginally. Anemia at birth is manifested as pallor, heart failure, or shock (Fig. 97-2). It may be due to acute or chronic fetal blood loss, hemolysis, or underproduction of erythrocytes. Specific causes include hemolytic disease of the newborn, tearing or cutting of the umbilical cord during delivery, abnormal cord insertion, communicating placental vessels, placenta previa or abruptio, nuchal cord, incision into the placenta, internal hemorrhage (liver, spleen, intracranial), α-thalassemia, congenital parvovirus infection or other hypoplastic anemias, and twin-twin transfusion in monozygotic twins with arteriovenous placental connections (Chapter 92).
Treatment of neonatal anemia by blood transfusion depends on the severity of symptoms, the hemoglobin level, and the presence of co-morbid diseases (bronchopulmonary dysplasia, cyanotic congenital heart disease, respiratory distress syndrome) that interfere with oxygen delivery. The need for treatment with blood should be balanced against the risks of transfusion, including hemolytic transfusion reactions, exposure to blood product preservatives and other potential toxins, volume overload, possible increased risk of retinopathy of prematurity and necrotizing enterocolitis, graft-versus-host (GVH) reaction, and transfusion-acquired infection (cytomegalovirus [CMV], HIV, parvovirus, hepatitis B and C) (Chapter 468). The risk of CMV infection can be almost eliminated by the use of leukoreduced blood. In the infant <1,500 g, CMV antibody-negative leukoreduced blood should be used. The risk of acquiring HIV and hepatitis B and C viruses is reduced but not eliminated by antibody screening of donated blood. Blood banking techniques that limit multiple donor exposure should be encouraged.
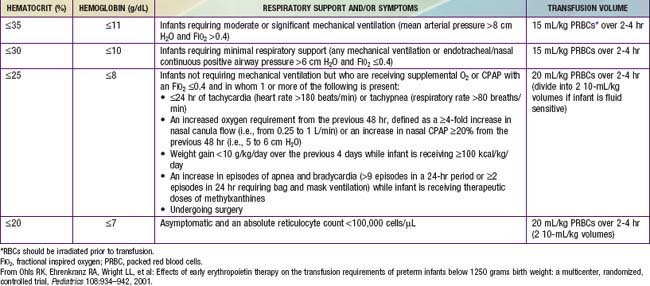
Although transfusion guidelines for preterm infants have been proposed (Table 97-2), they have not been subjected to rigorous clinical study. Nonetheless, these guidelines have led to a decline in the number of unnecessary transfusions. The use of restrictive vs more liberal transfusion guidelines has been examined in two randomized trials, one conducted at University of Iowa and a second multicentric trial known as the PINT (Premature Infants in Need of Transfusion) study. The restrictive guidelines in the two groups were generally similar. In the Iowa trial, the transfusion thresholds in the liberal- and restrictive-transfusion groups were <46% and <34%, respectively, in tracheally intubated infants receiving assisted ventilation; <38% and <28%, respectively, in infants receiving nasal continuous positive airway pressure or supplemental oxygen; and <30% and <22%, respectively, in infants breathing room air. The transfusion thresholds for the liberal groups were higher in the Iowa trial than in the PINT study. In both trials, the use of restrictive thresholds resulted in fewer transfusions and also increased the number of infants who received no transfusions at all. However, in the Iowa trial (but not in the PINT study), restrictive transfusion thresholds were associated with increases in major cranial ultrasonographic abnormalities and in the frequency of apneic spells. Although these findings need further evaluation in clinical studies, the issue of finding an appropriate transfusion threshold in premature infants remains unresolved.
Aher S, Ohlsson A: Late erythropoietin for preventing red blood cell transfusion in preterm and/or low birth weight infants, Cochrane Database Syst Rev (3):CD004868, 2006.
Anderson C. Critical haemoglobin thresholds in premature infants. Arch Dis Child Fetal Neonatal Ed. 2001;84:F146-F148.
Bell EF, Strauss RG, Widness JA, et al. Randomized trial of liberal versus restrictive guidelines for red blood cell transfusion in preterm infants. Pediatrics. 2005;115:1685-1691.
Bizzarro MJ, Colson E, Ehrenkranz RA. Differential diagnosis and management of anemia in the newborn. Pediatr Clin North Am. 2004;51:1087-1107.
Christensen RD, Henry E. Hereditary spherocytosis on neonates with hyperbilirubinemia. Pediatrics. 2010;125:120-125.
Ferguson D, Hébert PC, Lee SK, et al. Clinical outcomes following institution of universal leukoreduction of blood transfusions for premature infants. JAMA. 2003;289:1950-1956.
Hébert PC, Fergusson D, Blajchman MA, et al. Clinical outcomes following institution of the Canadian universal leukoreduction program for red blood cell transfusions. JAMA. 2003;289:1941-1949.
Hutton EK, Hassan ES. Late vs early clamping of the umbilical cord in full-term neonates. JAMA. 2007;297:1241-1252.
Kirpalani H, Whyte RK, Andersen C, et al. The Premature Infants in Need of Transfusion (PINT) study: a randomized, controlled trial of a restrictive (low) versus liberal (high) transfusion threshold for extremely low birth weight infants. J Pediatr. 2006;149:301-307.
Nicaise C, Gire C, Casha P, et al. Erythropoietin as treatment for late hyporegenerative anemia in neonates with Rh hemolytic disease after in utero exchange transfusion. Fetal Diagn Ther. 2002;17:22-24.
Ohlsson A, Aher SM: Early erythropoietin for preventing red blood cell transfusion in preterm and/or low birth weight infants, Cochrane Database Syst Rev (3):CD004863, 2006.
Rabe H, Reynolds G, Diaz-Rossello J: Early versus delayed umbilical cord clamping in preterm infants, Cochrane Database Syst Rev (4):CD003248, 2004.
97.2 Hemolytic Disease of the Newborn (Erythroblastosis Fetalis)
Hemolytic Disease of the Newborn Caused by Rh Incompatibility
Clinical Manifestations
A wide spectrum of hemolytic disease occurs in affected infants born to sensitized mothers, depending on the nature of the individual immune response. The severity of the disease may range from only laboratory evidence of mild hemolysis (15% of cases) to severe anemia with compensatory hyperplasia of erythropoietic tissue leading to massive enlargement of the liver and spleen. When the compensatory capacity of the hematopoietic system is exceeded, profound anemia occurs and results in pallor, signs of cardiac decompensation (cardiomegaly, respiratory distress), massive anasarca, and circulatory collapse. This clinical picture of excessive abnormal fluid in two or more fetal compartments (skin, pleura, pericardium, placenta, peritoneum, amniotic fluid), termed hydrops fetalis, frequently results in death in utero or shortly after birth. With the use of anti-D gamma globulin to prevent Rh sensitization, nonimmune (nonhemolytic) conditions have become frequent causes of hydrops (Table 97-3). The severity of hydrops is related to the level of anemia and the degree of reduction in serum albumin (oncotic pressure), which is due in part to hepatic dysfunction. Alternatively, heart failure may increase right heart pressure, with the subsequent development of edema and ascites. Failure to initiate spontaneous effective ventilation because of pulmonary edema or bilateral pleural effusions results in birth asphyxia; after successful resuscitation, severe respiratory distress may develop. Petechiae, purpura, and thrombocytopenia may also be present in severe cases as a result of decreased platelet production or the presence of concurrent disseminated intravascular coagulation.
Table 97-3 ETIOLOGY OF HYDROPS FETALIS*
| CATEGORY | DISORDER(S) |
|---|---|
| Anemia | Immune (Rh, Kell) hemolysis |
| α-Thalassemia | |
| Red blood cell enzyme deficiencies (glucose-6-phosphate dehydrogenase) | |
| Fetomaternal hemorrhage | |
| Donor in twin-to-twin transfusion | |
| Diamond-Blackfan syndrome | |
| Cardiac dysrhythmias | Supraventricular tachycardia |