Blistering and Pustular Diseases
Libby Edwards
Blisters are skin lesions that are filled with fluid. Blisters larger than half a centimeter are called bullae, and those smaller are called vesicles. Certainly, there is overlap between bullae and vesicles, but most vesicular diseases have primarily small blisters, and most bullous diseases have prominent larger blisters. Pustules are blister that are filled with pus, and these are nearly always small. Sometimes the identification of blisters and pustules is not straightforward. When blisters occur on fragile skin such as the genital area or any mucous membrane, the blister is unroofed quickly, leaving erosions so that the original blistering nature is not appreciated. Also, some blistering lesions are superficial and thus innately fragile, with the blister split occurring within the epidermis. Erosions resulting from a blister are usually well demarcated and round, or arcuate when blisters coalesce before eroding. Often, there are blisters on surrounding or extragenital skin that provide a clue to the blistering nature. As an intact vesicle ages, the fluid can appear cloudy and mimic a pustule. However, if the lesion is pierced, the fluid is clear rather than thick pus, showing the true vesicular nature.
Blistering Infections
Herpes Simplex Virus Infection
The classic genital vesicular disease is herpes simplex virus (HSV) infection, a lifelong sexually transmitted disease that is often asymptomatic but, when symptomatic, manifested by recurrent painful blisters and erosions. There are two types of HSV, type 1, which primarily produces oral infection, and type 2, which accounts for the vast majority of genital HSV infection. The Center for Disease Control reports that there is an increasing proportion of HSV 1 occurring on genital skin,1 presumably due to the frequency of oral sexual activity.
There are estimates of nearly half a billion people 0-49 years old worldwide with genital HSV infection in 2016, of whom only 122-192 million are caused by HSV 1.2 In the United States, there was an estimated prevalence of 18.6 million people between 18 and 49 years with HSV-2, with two-thirds of infections occurring in women.3 Older people are more likely to be seropositive than younger, and numbers are highest in Africa, with South Pacific, South Asia, and then the Americas next. In the United States, 12.1% of individuals age 14-49 were positive for HSV 2, down from 18% in 1999-2000.2 Highest numbers were in non-Hispanic Black patients (34.6%), and lowest in non-Hispanic Asians (3.8%), with almost twice as many women affected as men.2
Clinical Presentation
Herpes simplex virus infection is especially likely in younger, sexually active individuals, and in those who have higher risks of contagion due to several lifetime sexual partners.
Most people with genital HSV are unaware of their disease and experience latent disease. HSV produces both primary and recurrent infections. Primary infection with HSV is often subclinical and unrecognized, estimated to occur in about 80% of cases. When a primary infection is recognized, the outbreak is much more severe than recurrent episodes. Primary genital HSV infection follows exposure by 2-7 days and generally exhibits associated fever, malaise, headache, and other constitutional symptoms. Regional lymphadenopathy is usual; pain and edema may cause urinary retention. The lesions present as 1 to 3 mm, scattered and grouped vesicles occurring typically on the mucous membrane and modified mucous membrane portion of the vulva, sometimes extending to keratinized skin, or the glans or shaft of the penis in men (Figs. 10-1, 10-2, 10-3, 10-4, 10-5, 10-6, 10-7, 10-8, 10-9). Often, well-demarcated, discrete, round, and arcuate erosions in addition to or instead of vesicles are seen, because the blisters are fragile and quickly rupture. Healing requires about 2 weeks but may be complicated by irritation from overwashing or topical agents used empirically by an anxious patient.
After a primary infection, HSV remains latent in neuronal cells located in the dorsal ganglia. Then, the virus may intermittently reactivate, producing recurrent disease that is usually milder, localized, and shorter in duration. These recurrences are generally not associated with constitutional symptoms. However, patients experience a prodrome of localized tingling, burning, or dysesthesias before the onset of skin lesions. Recurrent HSV is also often located on the mucous membranes and modified
mucous membrane portion of the vulva, or the glans and shaft of the penis, but lesions can occur on any epithelial surface, including the buttocks, sacral area, scrotum, perianal skin, and hair-bearing labia majora. Rather than scattered discrete vesicles, recurrent episodes are characterized by a small area of grouped vesicles that quickly develop into well-demarcated, round, and arciform erosions. Round crusts rather than erosion are common on dry, keratinized skin such as the shaft of the penis or the hair-bearing labia majora. Rarely, recurrent HSV outbreaks can occur as fissures, most often in skinfolds, such as the interlabial sulci of the vulva or within normal skin wrinkling of the penile shaft.
mucous membrane portion of the vulva, or the glans and shaft of the penis, but lesions can occur on any epithelial surface, including the buttocks, sacral area, scrotum, perianal skin, and hair-bearing labia majora. Rather than scattered discrete vesicles, recurrent episodes are characterized by a small area of grouped vesicles that quickly develop into well-demarcated, round, and arciform erosions. Round crusts rather than erosion are common on dry, keratinized skin such as the shaft of the penis or the hair-bearing labia majora. Rarely, recurrent HSV outbreaks can occur as fissures, most often in skinfolds, such as the interlabial sulci of the vulva or within normal skin wrinkling of the penile shaft.
 Fig. 10-2. These oval erosions are typical of the remains of the fragile vesicles of HSV on delicate skin. |
 Fig. 10-3. Primary HSV is characterized by widespread discrete and coalescing erosions rather than a localized plaque of erosions or vesicles. |
Usually, the first recognized episode of a genital HSV infection has a recurrent pattern and course, since the primary infection is most often subclinical and, therefore, unrecognized. Because the appearance of this nonprimary but first clinical episode is delayed, the time and
circumstances of transmission cannot be determined; on occasion, this delay is years long.
circumstances of transmission cannot be determined; on occasion, this delay is years long.
 Fig. 10-4. These typical vesicles of recurrent HSV on the buttocks are on keratinized skin and tend to remain as blisters longer than those on thinner genital skin or mucosa. |
 Fig. 10-5. Older vesicles often appear pustular; however, if these are pierced, clear fluid is expressed, showing the true vesicular nature. |
Immunosuppressed patients sometimes are unable to contain their HSV infections. This is especially common in those with cell-mediated issues as occurs in human immunodeficiency virus disease and immunosuppressive medications (see Chapters 7 and 15). These patients are likely to experience chronic HSV that can become ulcerative rather than erosive, with ongoing enlargement of the area of involvement (Fig. 10-10). In addition, there is a synergistic effect of HSV and HIV, with the presence of HSV infection predisposing to HIV infection.
 Fig. 10-6. Sometimes these superficial erosions become macerated and lose their sharp definition that otherwise normally displays round erosions of HSV. |
Also, HSV has been shown to be a risk factor for bacterial vaginosis in women. And, the association with depression, loss of self-esteem, and shame is well recognized. A recent study shows that depression associated with HSV may even be higher than that associated with HIV.4
Diagnosis
The diagnosis of classic HSV is a clinical one, but laboratory confirmation is often important to the patient, since the diagnosis is psychologically crucial. The test of choice is polymerase chain reaction, with sensitivity of 80%-90%. A swab for direct fluorescent antibody is quick and is positive in 60%-70% of cases, but an adequate
sample is of utmost importance.5 Scraping the base of the ulcer with a number 15 blade usually suffices for an adequate cell sample, but the CDC reports that sensitivity is inadequate.1 A viral culture is only positive in about 50% of patients with HSV. Very sensitive is a shave skin biopsy from the edge of an erosion or of an intact vesicle (Fig. 10-11). However, a biopsy only diagnoses the presence of a herpes blister but does not differentiate HSV from VZV. That differentiation can generally be made clinically. The Tzanck preparation, although very quick, is less reliable, even in experienced hands, and again does not differentiate between HSV and VZV.1 Serology is not an adequate means of diagnosing an episode of HSV. Positive IgG for HSV varies very widely according to the country, occurring in up to 80% of adults; this denotes exposure, but not current, active disease. Negative serology indicates no past infection, but does not rule out a primary episode, since about 6 weeks is required for conversion.
sample is of utmost importance.5 Scraping the base of the ulcer with a number 15 blade usually suffices for an adequate cell sample, but the CDC reports that sensitivity is inadequate.1 A viral culture is only positive in about 50% of patients with HSV. Very sensitive is a shave skin biopsy from the edge of an erosion or of an intact vesicle (Fig. 10-11). However, a biopsy only diagnoses the presence of a herpes blister but does not differentiate HSV from VZV. That differentiation can generally be made clinically. The Tzanck preparation, although very quick, is less reliable, even in experienced hands, and again does not differentiate between HSV and VZV.1 Serology is not an adequate means of diagnosing an episode of HSV. Positive IgG for HSV varies very widely according to the country, occurring in up to 80% of adults; this denotes exposure, but not current, active disease. Negative serology indicates no past infection, but does not rule out a primary episode, since about 6 weeks is required for conversion.
 Fig. 10-8. Very mild or early HSV can be very subtle and show only red papules without clinical vesicles. However, a biopsy shows microscopic changes of HSV. |
The histologic appearance of HSV includes an intraepidermal vesicular dermatitis formed by acantholysis. Individual keratinocytes show intracellular edema (ballooning) and reticular degeneration (intracellular edema causing cell walls to burst). Eosinophilic intranuclear inclusion bodies may be present. Multinucleated keratinocytes are pathognomic for herpes virus infections but do not distinguish between HSV and varicella-zoster virus (VZV) infections.
HSV must be differentiated from several other vesicular and pustular diseases. Occasionally, the differentiation of genital HSV infection from VZV infection can be difficult, because both exhibit grouped vesicles or erosions. However, genital VZV infection usually occurs in the older patient and presents in a dermatomal pattern, also affecting the medial thigh or buttock unilaterally. VZV infection occurs once in the immunocompetent patient, rather than recurrently, as is usual with HSV infection. The fragile pustule roofs of Candidiasis of the modified mucous membranes of the vulva or the uncircumcised glans penis sometimes mimic the erosions of HSV. A fungal smear is positive in that case. Scattered discrete red papules, pustules, or crusts of folliculitis, both irritant and
staphylococcal, can resemble primary HSV. Erythema multiforme and Stevens-Johnson syndrome both mimic and sometimes follow HSV infection. A hypersensitivity reaction to HSV is the most common cause of recurrent erythema multiforme. These patients with blistering erythema multiforme usually exhibit both intraoral and genital erosions that are less well demarcated than those of HSV, and HSV does not occur inside the mouth, except for the initial primary outbreak. In addition, erythema multiforme generally also exhibits red, nonscaling, flattopped papules or even blisters on the palms, soles, and sometimes other keratinized surfaces is common. Finally, a fixed drug eruption can mimic herpes simplex virus infection by producing same-site recurrent blisters or erosions, but the lesions are generally larger rather than showing small coalescing vesicles or erosions.
staphylococcal, can resemble primary HSV. Erythema multiforme and Stevens-Johnson syndrome both mimic and sometimes follow HSV infection. A hypersensitivity reaction to HSV is the most common cause of recurrent erythema multiforme. These patients with blistering erythema multiforme usually exhibit both intraoral and genital erosions that are less well demarcated than those of HSV, and HSV does not occur inside the mouth, except for the initial primary outbreak. In addition, erythema multiforme generally also exhibits red, nonscaling, flattopped papules or even blisters on the palms, soles, and sometimes other keratinized surfaces is common. Finally, a fixed drug eruption can mimic herpes simplex virus infection by producing same-site recurrent blisters or erosions, but the lesions are generally larger rather than showing small coalescing vesicles or erosions.
HERPES SIMPLEX VIRUS INFECTION Diagnosis
Presentation
Primary, first episode HSV with scattered and grouped vesicles and round erosions on genital and perianal skin
Recurrent with grouped vesicles or erosions
Prodrome of tingling or burning
Confirmation by polymerase chain reaction technique or biopsy
Pathophysiology
Herpes simplex virus (HSV) types 1 and 2 are doublestranded DNA viruses (Herpesvirus hominis) capable of producing vesicular and erosive mucocutaneous disease. Genital HSV infection is usually produced by HSV type 2, while oral mucocutaneous and ocular disease are produced by HSV 1, despite the frequency of oral sexual activity. Essentially all HSV 2 is transmitted via genital-to-genital contact, whereas HSV 1 is transmitted by oral to genital contact, through saliva as well as direct contact with active oral lesions. Preexisting oral HSV 1 is protective against the acquisition of genital HSV 1, but not against contagion of HSV 2. Fortunately, genital disease produced by HSV 1 is less severe and less frequently recurrent that that produced by HSV 2, and neonatal HSV is less problematic in women with HSV 1.
Estradiol is known to be protective against the acquisition of HSV2, which is surprising since this infection is twice as common in men as women; a recent study suggest that the mechanism of action may be an enhanced establishment of antiviral memory T-cell responses in the presence of estradiol when exposed to HSV.6 However, there is evidence that some hormonal contraceptives, especially depot medroxyprogesterone acetate, may increase the risk of HSV acquisition.7
Management
The management of HSV infection involves far more than prescribing antiviral medication, and ideally would begin with prevention and education before exposure.8 However, from a practical standpoint, this begins with the sensitive and nonjudgmental education and counseling of the patient. This is not a diagnosis that should be given by a nonmedical office worker or by email. These patients exhibit marked anxiety, depression, fear, and self-esteem issues. There are little data on the best means for the management of the psychological effects of the diagnosis of genital HSV infection, but counseling and support should be provided or arranged.
The patient should be informed regarding the infectivity of the disease, its recurrent nature, and the importance of avoidance of sexual intercourse when open lesions are present. However, patients also should be counseled that intermittent shedding of the virus occurs when there is no evidence of active HSV infection, and the infection can be transmitted even in the absence of blisters and erosions. In fact, most HSV infection is contracted during asymptomatic episodes. Shedding also occurs in those receiving long-term suppressive antiviral medication, albeit in smaller amounts. Circumcision and condoms have been shown to confer modest protection from infection. Therefore, the use of a condom, even when there are no obvious lesions, is wise and confers significant but not complete protection.9 Neonatal transmission of HSV during delivery should be discussed to ensure the protection of future infants, although most neonatal HSV infections occur due to maternal primary infection, or at least recent acquisition.
HSV infections are shortened by prompt treatment with specific oral antiviral agents.1 There are three very equally effective antiviral choices for HSV. Primary HSV infection can be treated with acyclovir 400 mg three times a day, valacyclovir 1 g twice a day, or famciclovir 250 mg twice a day for 7-10 days, and the duration can be extended if lesions have not healed. A mild initial infection does not predict mild recurrences, and some patents will experience severe future outbreaks. All patients should be prepared for recurrences, at least with prescriptions, and even better with prescriptions that have been filled. Medication should be started with the prodrome, or at least with the first sign of lesions. Recurrences can be treated with acyclovir 800 mg 3 times a day for 2 days or 800 mg 2 times a day for 5 days; famciclovir 1 g twice a day for 1 day or 500 mg once followed by 250 mg twice a day for 2 days, or 125 mg twice daily for 5 days; or valacyclovir 500 mg for 3 days or 1 g daily for 5 days. Side effects are minimal with each. Topical antiviral agents provide minimal benefit.
Suppressive therapy with chronic oral antiviral agents is often used for patients who experience frequent recurrences and to decrease shedding and to minimize, but not
eliminate, the risk of transmission. Regimens for those with genital HSV 2 include acyclovir 400 mg twice daily, valacyclovir 500 mg or 1 g daily, or famciclovir 250 mg twice daily.1 Recurrences with genital HSV 1 are much less frequent with genital HSV 1, and after the first year, asymptomatic shedding is far less, so that suppressive therapy is often not required.
eliminate, the risk of transmission. Regimens for those with genital HSV 2 include acyclovir 400 mg twice daily, valacyclovir 500 mg or 1 g daily, or famciclovir 250 mg twice daily.1 Recurrences with genital HSV 1 are much less frequent with genital HSV 1, and after the first year, asymptomatic shedding is far less, so that suppressive therapy is often not required.
Intravenous acyclovir 5-10 mg/kg every 8 hours until clinical resolution occurs is occasionally needed if disease is severe, or there is organ involvement, especially if the patient is immunosuppressed or if gastrointestinal absorption is a major problem, as can occur in the HIV-positive patient. Resistance of HSV to currently available oral medications is rare and occurs primarily in immunocompromised patients, and the following are alternative treatments.1 Therapies include foscarnet 40-80 mg/kg IV every 8 hours until clinical resolution occurs is first-line therapy. Cidofovir 5 mg/kg IV weekly is also used. Both medications are nephrotoxic and should be administered by infectious disease specialists. Imiquimod 5% applied to the lesion for 8 hours 3 times/week has been reported to be effective as has compounded cidofovir gel 1% applied 2-4 times daily; however, cidofovir must be compounded at a pharmacy.
Therapeutic vaccines have been studied for years with many results in the literature, and COVID 19 has introduced new ideas and methods with use of mRNA vaccines. Some vaccines have shown promise, with high neutralizing titers and robust B-cell immune response.10,11
Some patients erroneously believe that HSV infection can be followed by postherpetic neuralgia. Fortunately, that is not a sequel to this infection; this only follows herpes zoster infection. Primary HSV infection can produce malaise and fever, and sometimes outbreaks can create radicular pain. Very rarely, HSV can cause HSV encephalitis.
HERPES SIMPLEX VIRUS INFECTION Management
Patient education
Antiviral therapy—primary
Acyclovir 400 mg tid for 7-10 days
Valacyclovir 1 g bid for 7-10 days
famciclovir 250 mg tid for 7-10 days
Antiviral therapy—recurrences
Acyclovir 800 mg bid for 5 days, or 800 mg tid for 2 days
Valacyclovir 1 g daily for 5 days or 500 mg bid for 3 days
Famciclovir 1 g bid for 1 day, or 500 mg once, then 250 mg bid for 2 day, or 125 mg bid for 5 days
Suppression of HSV 2:
Acyclovir 400 mg bid
Valacyclovir 500 mg to 1 g daily
Famciclovir 250 mg bid
Herpes Zoster Infection (Varicella, Shingles)
Varicella zoster virus (VZV) is a virus that is related to HSV, produces blisters that are similar morphologically, and lies latent in nerve ganglia like HSV. However, this virus causes two diseases distinct from those produced by HSV; the primary infection of scattered discrete vesicles, varicella, and herpes zoster, with grouped vesicles along a dermatome. Both can affect the genitalia but present very differently. The first episode of VZV (varicella, chicken pox) is rare in Western countries because of the availability of a very effective vaccine and presents with generalized rash. The subsequent episode of VZV, herpes zoster, is localized and painful. Hopefully, with the availability of both the varicella and shingles vaccines, these conditions will become vanishingly rare.
Clinical Presentation
The initial infection with VZV is varicella (chickenpox). This usually occurs in childhood and is extraordinarily infectious, so that in the past, nearly all adults had had varicella, whether or not it was remembered. Now, in most industrialized countries, adults over 30 years were vaccinated as children, and childhood varicella is rare. Varicella presents with fever and constitutional symptoms, then a generalized pruritic eruption of vesicles with a predilection for the genital skin and vagina. Lesions begin as small red papules, then evolve into vesicles that develop the central depression that is characteristic of a herpetic vesicle, and then a central crust. Multiple stages of these lesions simultaneously are characteristic (Fig. 10-12). Mucous membranes are often involved.
 Fig. 10-12. Varicella is mostly a childhood disease and manifested by red papules, vesicles, and crusts that include both keratinized skin and mucous membranes. |
 Fig. 10-13. Clustered and coalescing vesicles on a red base, distributed along a dermatome are characteristic of herpes zoster. |
The reactivation of the latent varicella zoster virus, herpes zoster, occurs primarily in patients with immune deficiency by virtue of age, disease, or medications, but occasionally occurs in young and healthy individuals. Constitutional symptoms are minimal or absent compared to varicella, but many patients experience a localized prodrome of pain, itching, burning, or aching, followed in one or a few days by pink, nonscaling papules and plaques that resemble hives in a unilateral dermatomal distribution (Figs. 10-13 and 10-14) in the symptomatic area. Blisters they form on the pink plaques, and coalesce into larger blisters. Hemorrhage into the blisters later give a purple, purpuric color to some blisters (Fig. 10-15). Then, the blisters crust and heal over the next 2 or 3 weeks. The surface of fragile blisters on mucous membranes and modified mucous membranes of the genitalia are quickly unroofed so that erosions usually predominate in those areas. Likewise, the friction from skinfolds and clothing often tears blister roofs even on the more keratinized genital skin. Herpes zoster can become disseminated, ulcerative, chronic, or hyperkeratotic in immunosuppressed patients (see Chapter 15).
 Fig. 10-14. Like HSV, herpes zoster is generally preceded by a prodrome of pain in the distribution of the skin lesions. |
Herpes zoster is sometimes associated with mild headache, malaise, and a low-grade fever. But, the primary complication of herpes zoster infection is the development of postherpetic neuralgia, in which chronic pain persists after the eruption resolves. This is most common in individuals over 60 years of age and in immunosuppressed people. Unlike HSV, VZV does not typically recur in immunocompetent patients, and most patients with “recurrent shingles” of the genitalia actually experience recurrent HSV. Although some providers believe that postherpetic neuralgia is responsible for vulvodynia, penodynia, and scrotodynia, historically herpes zoster rarely is reported as a preceding event. There is now a suggestion that vaccination with COVID 19 may precipitate the appearance of herpes zoster.12
Diagnosis
The diagnosis of herpes zoster is usually made clinically, on the basis of plaques of blisters distributed along dermatomes. When necessary, this can be confirmed by the identification of viral DNA with the PCR technique. The virus is rather fastidious, so cultures are sometimes falsely negative, but the PCR technique is very sensitive and now easily available when performed in the first 48-72 hours. A Tzanck preparation is poorly validated and does not differentiate HSV from
VZV. A biopsy is extremely sensitive but also does not differentiate between HSV infection and herpes zoster, a distinction that can usually be made on clinical grounds.
VZV. A biopsy is extremely sensitive but also does not differentiate between HSV infection and herpes zoster, a distinction that can usually be made on clinical grounds.
The histologic appearance of herpes zoster infection is identical to that of HSV infection. Keratinocytes swell and burst, and some epithelial cells form giant cells. Occasionally, leukocytoclastic vasculitis underlies these epithelial changes, but this does not indicate a primary or possibly systemic problem of vasculitis.
Occasionally, genital herpes zoster can be difficult to differentiate from HSV. However, HSV is a recurrent disease, whereas herpes zoster is a one-time event, except in significantly immunosuppressed patients. Both diseases can be unilateral, and both are classically painful. Other blistering diseases should be considered, such as bullous impetigo, but these usually show larger blisters, are not generally grouped, are not especially painful, and not dermatomal in distribution.
HERPES ZOSTER VIRUS INFECTION Diagnosis
Morphology of painful plaques of clustered vesicles in a dermatomal distribution
Confirmation by PCR if needed
Pathophysiology
Herpes zoster infection is a blistering disease that results from reactivation of VZV that has been latent in nerve ganglia since a remote episode of varicella. This condition occurs most often in older individuals whose immune system is less efficient or in immunocompromised patients.
Management
Even more important than antiviral therapy is pain control for most patients. Narcotic pain analgesia is needed in some patient for the first few weeks. Only very early diagnosis and treatment (within 72, but especially 48 hours) with oral antiviral therapy somewhat reduces the duration of an acute infection. This improvement is not striking, so therapy is optional in younger, healthy patients. Treatment after this time does not affect the short-term or long-term course of the disease. However, immunosuppressed or elderly people should be treated. Some studies have shown an arguably significant reduction of postherpetic neuralgia in patients treated early.
Choices include acyclovir 800 mg five times a day for 7-10 days, famciclovir 500 mg three times daily for 7 days, and valacyclovir 1 g every 8 hours for 7 days. The advantage of valacyclovir and famciclovir is the less frequent dosing schedule.
Those patients who experience postherpetic neuralgia deserve medication for neuropathic pain, including amitriptyline, gabapentin, pregabalin, venlafaxine, or duloxetine, the same medications as used for genital pain syndromes and discussed in Chapter 13. Sometimes, referral to a pain clinic is necessary. These medications can be started early, since several weeks are required for onset of relief.
Otherwise, the best current management is prevention. A two dose effective vaccine for the prevention of herpes zoster is available, approved by the Food and Drug Administration for people over 60 years of age.
HERPES ZOSTER VIRUS INFECTION Management
Patient education
Antiviral therapy
Acyclovir 800 mg 5×/day for 7-10 days
Valacyclovir 1 g q8h for 7 days
Famciclovir 500 mg tid for 7days
Pain control
Attention to post herpetic neuralgia
Impetigo
Impetigo is a bacterial infection of the superficial epidermis that produces blisters, crusting, and erosions. Some phage types of Staphylococcus aureus cause separation of the stratum corneum from the rest of the epidermis and very fragile blisters, with rapid loss of this fragile blister roof. Then, well-demarcated, superficial round erosions with collarettes are typical (Fig. 10-16). When produced by α-hemolytic Streptococcus infection, the classic honey
crusting and erosions commonly occur. Bacterial folliculitis often accompanies impetigo, where infection that has extended into nearby hair follicles, producing discrete red papules, pustules, and small erosions and crusts. The diagnosis is confirmed by a bacterial culture and response to therapy. A culture is important due to the frequency of resistant S aureus. Treatment consists of an antibiotic effective against both S aureus and Streptococcus species while awaiting culture results, such as cephalexin, clindamycin, and trimethoprim-sulfamethoxazole. Despite prompt response to therapy, lesions sometimes recur after therapy. This occurs most often in patients who are nasal carriers of S aureus, and these patients benefit from the administration of intranasal mupirocin cream or ointment four times a day, for 1 week each month for several months to minimize this carrier state.
crusting and erosions commonly occur. Bacterial folliculitis often accompanies impetigo, where infection that has extended into nearby hair follicles, producing discrete red papules, pustules, and small erosions and crusts. The diagnosis is confirmed by a bacterial culture and response to therapy. A culture is important due to the frequency of resistant S aureus. Treatment consists of an antibiotic effective against both S aureus and Streptococcus species while awaiting culture results, such as cephalexin, clindamycin, and trimethoprim-sulfamethoxazole. Despite prompt response to therapy, lesions sometimes recur after therapy. This occurs most often in patients who are nasal carriers of S aureus, and these patients benefit from the administration of intranasal mupirocin cream or ointment four times a day, for 1 week each month for several months to minimize this carrier state.
Noninfectious Blistering Eruptions
Blisters that are not produced by infection are usually caused by autoimmune disease, hypersensitivity reactions, or chemical or thermal burns, but Hailey-Hailey disease results from a genetic abnormality in the structure of the skin.
In autoimmune blistering diseases, autoantibodies are directed against target antigens that are involved in helping epithelial cells adhere to each other or to the basement membrane. Such antibodies fix to the target antigen and trigger complement activation that eventuates in destruction of the adherence of these structures to each other, resulting in blisters. The fragile blisters of the mucous membranes and modified mucous membranes are so transient as to be unnoticed, and the usual presentation is that of erosion. However, the round shapes of the erosions and the frequent accompanying blisters on nearby keratinized skin often declare the underlying blistering nature of the disease.
A biopsy is important for the correct diagnosis of noninfectious blistering diseases. The best site for biopsy is the edge of a blister on nonmucous membrane skin, so that both normal skin and the blister are visualized, and the location of the blister within the skin is seen. The sample is placed in formalin for a routine biopsy. The edge of an erosion often gives the diagnosis if an intact blister is not present. If there are no intact blisters on keratinized skin, then the edge of an erosion or mucous membrane blister can be sampled. When an immunobullous disease is considered, nearby normal skin should be sampled and placed in transport media for direct immunofluorescent testing.
Pemphigus
Clinical Presentation
Pemphigus comprises a group of associated autoimmune superficial blistering diseases that affect the skin and mucous membranes. There are three major types of pemphigus; pemphigus vulgaris, making up about 70% of all patients with pemphigus; pemphigus foliaceous, which does not exhibit mucous membrane involvement; and paraneoplastic pemphigus. This discussion is directed toward pemphigus vulgaris. Pemphigus vulgaris (vulgaris, meaning common) occurs most often between the ages of 30 and 60 years, most frequently in women, and more common in the Ashkenazi Jewish population and Mediterranean descendants.13 Childhood cases are rare.
Most patients with pemphigus vulgaris present with painful oral erosions that are not recognized as blisters. Blisters on extramucosal skin may not follow for several months. Nearly all patients with pemphigus vulgaris experience mucosal disease at some point. Forty-one percent of patients experience genital involvement, and 35% of women exhibit Pap smears that report acantholysis and inflammation consistent with but not diagnostic of pemphigus,14 and genital blisters and erosions are common.
Pemphigus vulgaris occurs on keratinized skin, as well as on all squamous mucous membrane and modified mucous membrane sites. Blisters of pemphigus are notoriously superficial and fragile, so that clinical bullae are rarely recognized mucous membranes. Instead, rather bland, nonspecific erosions are often the lesions first noted (Fig. 10-17, 10-18, 10-19). Pemphigus is classically a nonscarring disease, but the vulva scars easily so that resorption of the labia minora is common, and clitoral phimosis can occur, and the end stage mimics lichen planus, lichen sclerosus, and mucous membrane pemphigoid.
Pemphigus vulgaris of the penis is less common than pemphigus of the vulva, and when present it occurs most often on the glans, but the distal shaft and corona also
are sites that are affected. Scarring of the penis can occur, with blunting of the corona in circumcised men and phimosis in uncircumcised individuals. The oral mucosa is usually affected in both men and women (Fig. 10-20).
are sites that are affected. Scarring of the penis can occur, with blunting of the corona in circumcised men and phimosis in uncircumcised individuals. The oral mucosa is usually affected in both men and women (Fig. 10-20).
 Fig. 10-17. The blisters of pemphigus vulgaris are fragile, so that intact fluid-filled bullae are often not seen. Here, there are collapsed lesions with flaccid necrotic blister roofs and erosions. |
The blisters of pemphigus on keratinized skin are superficial and flaccid. Gentle traction on a blister extends the blister by shearing the tenuous adherence of the upper epidermis to underlying tissue, producing the Nikolsky sign. These very superficial blisters rupture easily to leave large eroded areas, but they normally heal without scarring except on the genitalia.
 Fig. 10-20. Pemphigus regularly affects the mouth as well, and like the genital skin, erosions rather than intact blisters are the rule. However, small vesicles can be seen around the nose. |
Pemphigus vegetans is a variant of pemphigus vulgaris characterized by erosions, often with peripheral pustules in the early phases of the disease. Later, large, thickened, and frequently verrucous plaques occur (Fig. 10-21).
Pemphigus foliaceous is a rare condition that generally begins on the chest, scalp, and face, and not recognized as a blistering condition because of the very superficial nature and tiny size of the blisters. These do not generally affect mucous membranes. Paraneoplastic pemphigus has many different clinical presentations but typically presents with painful mucosal erosions and dusky patches on the skin that later desquamate. Severe widespread disease
can result in dehydration requiring intensive care management or a burn center, and genital involvement is a relatively minor issue. This disease is often systemic rather than affecting only epithelial surfaces, generally fatal, and is usually associated with lymphoproliferative neoplasms.15
can result in dehydration requiring intensive care management or a burn center, and genital involvement is a relatively minor issue. This disease is often systemic rather than affecting only epithelial surfaces, generally fatal, and is usually associated with lymphoproliferative neoplasms.15
 Fig. 10-21. Pemphigus vegetans is an uncommon manifestation of pemphigus, where erosions are covered with thickened hyperkeratotic plaques. |
Diagnosis
All variants of pemphigus are diagnosed by skin biopsy, although the clinical morphology of fragile bullae in a setting of mucous membrane erosions is highly suggestive. The skin biopsy shows a superficial blister within the epidermis (Fig. 10-22). Pemphigus vulgaris and early pemphigus vegetans exhibit a blister located just above the basal cell layer. The basal cells adhere to the basement membrane, but not to each other or to overlying cells, thus producing a picture similar to that of a row of tombstones. In addition, within the blister cavity are detached epidermal cells that, with loss of cohesion to surrounding cells, appear round and are called acantholytic cells. Direct immunofluorescence biopsies obtained from normalappearing skin near a blister or erosion show immunoglobulin G (IgG) deposition in the epidermal intercellular substance. In addition, indirect immunofluorescence of patients’ serum showing IgG autoantibodies that bind to the epidermal cell surface occur in 70%-90% of patient with PV.13
Biopsies of pemphigus vegetans often show, in addition, neutrophilic inflammation producing intraepithelial abscesses. In its later, hyperkeratotic form, pemphigus vegetans reveals squamous hyperplasia and pseudoepitheliomatous hyperplasia on biopsy, in addition to the characteristic suprabasilar blister. Pemphigus foliaceus shows a very superficial blister formed by acantholysis in the uppermost epidermis.
Most other blistering diseases can mimic PV. Pemphigus vulgaris often begins with mucosal erosions indistinguishable from erosive lichen planus. Routine biopsies usually differentiate the two diseases, and pemphigus vulgaris progresses to produce blisters and erosions on keratinized skin, unlike lichen planus. Pemphigus vulgaris resembles mucous membrane pemphigoid early on, with both producing nonspecific mucosal erosions. The milder and intermittent occurrence of a fixed drug eruption and the explosive onset of blistering forms of erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis usually distinguish these diseases from pemphigus.
PEMPHIGUS VULGARIS Diagnosis
Morphology of mucosal erosions, flaccid blisters of dry, keratinized skin
More gradual onset compared to Stevens-Johnson syndrome and toxic epidermal necrolysis
Confirmation by routine and direct immunofluorescent biopsies
Pathophysiology
Pemphigus is a group of autoimmune intraepidermal bullous diseases produced by IgA autoantibodies to the surface of epidermal cells. The IgG is directed primarily against desmogleins 1 and 3, proteins that function in the attachment of squamous cells to each other, but other autoantibodies are often present as well, perhaps providing a synergistic effect.13
This ultimately produces a loss of adhesion of these cells, leading a superficial blister within the epidermis. The most common form of pemphigus, pemphigus vulgaris, is produced by loss of adhesion of the basal cells from the upper epidermis.
There is a genetic component to PV also, with certain major histocompatibility complex (MHC) class II molecules occurring more frequently in individuals with PV. These are the same MHC seen more often in the Jewish population and in countries of the eastern Mediterranean and the Middle East and borne out in the epidemiology of the disease. Like pemphigus vulgaris, pemphigus vegetans is produced by a split in the epidermis just above the basal cell layer, but it is characterized by the later development of thickened, hyperkeratotic skin.
In some cases, medications can precipitate pemphigus. The most common medication is penicillamine, and other offenders are captopril, tiopronine and related drugs, as well as nonsteroidal anti-inflammatory medications, and calcium channel blockers.16 As many as 7% of patient taking penicillamine for 6 months can develop
PV.17 Injury has also precipitated pemphigus at times. One form of pemphigus, benign familial pemphigus (also called Hailey-Hailey disease), is of autosomal dominant rather than autoimmune origin, and it is discussed later in this chapter.
PV.17 Injury has also precipitated pemphigus at times. One form of pemphigus, benign familial pemphigus (also called Hailey-Hailey disease), is of autosomal dominant rather than autoimmune origin, and it is discussed later in this chapter.
Management
Pemphigus generally is rarely controlled with topical therapy, although the genitalia benefit from local care. This widespread disease is treated systemically, falls in the prevue of a dermatologist, and ideally includes careful evaluation of the patients’ overall health, and recruitment of a primary care provider, with availability of other specialists such as dentists and ophthalmologists to aid with management of common issues with the disease and side effects of medications.18 Comorbidities of hypertension, diabetes, obesity, osteoporosis, etc. are common due to therapy, and these should be anticipated and managed.19
Specific first-line therapy for pemphigus is now systemic corticosteroids and CD20 inhibitors, specifically, rituximab infusions, now approved by the Food and Drug Administration for pemphigus. The advent of systemic corticosteroids has been lifesaving in these patients who generally died before the use of corticosteroids.20 Now, the cause of death in patients with pemphigus now is primarily pneumonia and septicemia due to therapy.21 Initial therapy is prednisone or its equivalent, beginning at about 40-80 mg/d, and adjusting the dose up or down as needed. Pemphigus is not a disease that is curable, so control is the goal. This condition is chronic, and patients generally are on prolonged courses of systemic corticosteroids. However, the newer use of rituximab has been a remarkable addition to the management of pemphigus, often allowing patients to discontinue prednisone. This medication eliminates the antibodies to desmogleins by depleting B cells, and it may be more effective if begun early in the course of the disease. After initial dosing, patients are usually maintained with semiannual infusions.
Second-line therapy consists of the steroid-sparing agents azathioprine and mycophenolate mofetil, followed by intravenous immunoglobulin infusions, cyclophosphamide and immunoadsorption.13,18,19
Local care of the genital area is important for comfort and to prevent scarring that interferes with sexual functioning and micturition. Topical corticosteroid therapy in addition to systemic therapy may be useful. Intralesional corticosteroids are a time-honored safe and costeffective therapy for persistent erosions (see Chapter 4). Intralesional rituximab also has been reported as useful.22 One study of 11 patients treated recalcitrant pemphigus with two intralesional injections of biosimilar rituximab.23 There was a decrease in serum antibodies and B cells with this intralesional therapy, as well as healing of lesions, and there was a safer profile compared to intravenous infusions. One trial reported equivalent results of intralesional triamcinolone acetonide compared to intralesional rituximab.24
Measures to prevent secondary infection of genital skin, especially Candidal, are also important. Women especially who are on systemic corticosteroids should be monitored for this, and perhaps treated with weekly fluconazole, particularly when antibiotics are added or they are diabetic. Unlike many skin diseases in men, pemphigus is not cleared with circumcision and requires medical therapy. Women with vaginal involvement should insert dilators regularly to prevent vaginal adhesions, and uncircumcised men should retract the foreskin daily and apply petroleum jelly to prevent phimosis.
Although the prognosis of pemphigus vulgaris has improved remarkably since the use of corticosteroids and now rituximab, recurrence or low level persistence is common.
PEMPHIGUS VULGARIS Management
Prednisone 40-80 mg/d initially and rituximab infusions
Steroid-sparing adjuvant medications in addition when needed
Mycophenolate mofetil
Azathioprine
IVIG
Cyclophosphamide
Immunoadsorption
Local care to prevent scarring
Bullous Pemphigoid
Clinical Presentation
Although bullous pemphigoid is the most common autoimmune blistering disease, it does not generally affect mucous membranes or modified mucous membranes, so does not present primarily as a genital dermatosis. It occurs equally in men and women and is most common in individuals over 60 years of age, with no racial predilection. However, bullous pemphigoid rarely occurs in childhood. About 10% of patients experience genital involvement, primarily of keratinized epithelium rather than the mucous membranes.
Patients regularly complain of itching, and this often precedes the blisters, sometimes by months. The pruritic skin sometimes mimics urticaria, and eventually small vesicles and then bullae arise. The genital areas most likely to be involved are the hair-bearing areas of inner thighs, inguinal crease, and perineum. Blisters are tense and filled with straw-colored and, occasionally, hemorrhagic fluid (Figs. 10-23, 10-24, 10-25). Scarring does not occur in the absence of a complicating event such as secondary infection, since bullous pemphigoid does not affect mucous membranes.
 Fig. 10-23. Unlike pemphigus vulgaris, bullous pemphigoid exhibits tense blisters. (Courtesy of Errol Craig, MD.) |
An association of bullous pemphigoid with neurologic disease, especially dementia and Parkinson disease, has been described,25 but the long debated association of malignancy with bullous pemphigoid seems to be losing favor. There is a controversial association of bullous pemphigoid with underlying malignancy.26,27
Diagnosis
The clinical presentation of bullous pemphigoid allows for a provisional diagnosis, but confirmation by biopsy for routine histology and direct immunofluorescence is mandatory. Biopsy of an intact new blister shows characteristic histology; subepidermal blistering with a variable dermal inflammatory infiltrate including eosinophils is usual (Fig. 10-26). Direct immunofluorescent biopsy of perilesional skin shows deposition of IgG and complement components along the basement membrane zone (BMZ). A direct immunofluorescent examination of saltsplit skin shows that the binding is epidermal, to the roof of the blister. This differentiates bullous pemphigoid from epidermolysis bullosa acquisita, in which the binding is to the base or dermal side of the blister. Indirect immunofluorescent studies of the sera of patients with bullous pemphigoid show the presence of IgG directed toward the pemphigoid target antigens in the lamina lucida of the BMZ.
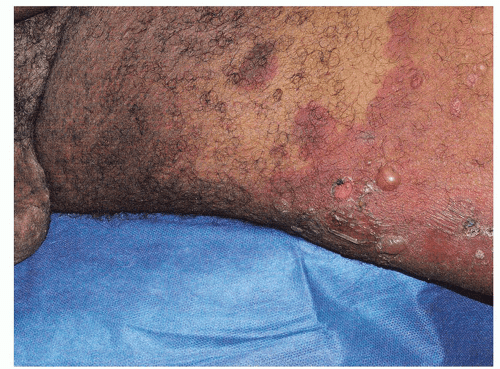 Fig. 10-24. Bullous pemphigoid affects the keratinized skin, generally sparing the mucous membranes and modified mucous membranes of the genitalia. |
 Fig. 10-25. The blisters of bullous pemphigoid occur from a deeper cleft in the skin, under the epidermis, resulting in tense, straw-colored blisters, rather than flaccid lesions. |
Most other blistering disease can be differentiated by the morphology and onset, but epidermolysis bullosa acquisita and linear IgA dermatosis can be difficult to distinguish without biopsies. Other blistering diseases to consider include Stevens-Johnson syndrome and toxic epidermal necrolysis, but these essentially always exhibit mucosal lesions, and the onset is generally more abrupt. Pemphigus vulgaris, unlike bullous pemphigoid, generally affects mucous membranes prominently and is characterized by erosions and flaccid bullae, rather than by
tense blisters. Less often, bullous impetigo can mimic bullous pemphigoid. However, blisters are usually few in number and flaccid and of sudden and recent onset. Mucous membranes are spared.
tense blisters. Less often, bullous impetigo can mimic bullous pemphigoid. However, blisters are usually few in number and flaccid and of sudden and recent onset. Mucous membranes are spared.
Pathophysiology
The blisters of bullous pemphigoid are produced by autoantibodies directed basal keratinocyte hemidesmosomal proteins BP antigen 230 (BPAG1) and BP antigen 180 (BPAG2 or type XVII collagen), which are crucial in the adhesion of the epithelium to the dermis. The binding of these autoantibodies is followed by inflammation and complement activation, ultimately with damage to the dermal-epidermal junction and detachment of the epidermis.28 Although usually occurring spontaneously and without an identifiable precipitating factor, bullous pemphigoid is sometimes associated with specific drugs, such as loop diuretics, aldosterone antagonists, dipeptidyl dipeptidase inhibitors, and PD1 and PDL-1 inhibitors.29
Management
For localized and prebullous disease, treatment with potent topical steroids sometimes is sufficient, especially in the elderly and those for whom systemic corticosteroids are especially dangerous. For generalized bullous pemphigoid, systemic steroids are first-line therapy. However, the doses required are generally from 40 to 60 mg/d, tapered according to response. Steroid-sparing immunosuppressive agents, whose effect is more delayed and less effective, are usually added; these include azathioprine, cyclophosphamide, or minocycline with nicotinamide. Methotrexate has also been used as a steroid-sparing agent. More recently, the biologic medications, including omalizumab, have been used and appear more beneficial.32,33 Rituximab has shown efficacy, but less than for pemphigus, and one study suggests that it is equivalent to omalizumab.33,34 Especially interesting seems the efficacy of dupilumab, which is only FDA approved in the United States for atopic dermatitis but has been shown useful in a number of reports for bullous pemphigoid and has a very good safety profile.35,36
The lesions in bullous pemphigoid are nonscarring, and the course of bullous pemphigoid is self-limiting, with remission in treated patients usually occurring in months to 6 years. Partly because bullous pemphigoid occurs primarily in the elderly, and because of the toxicity of therapy, the overall mortality of bullous pemphigoid in the first year or two after diagnosis is about 7 times the reference population.37 Although treatment does not significantly improve mortality, it improves the quality of life, which can be ruined by the intractable and miserable pruritus. The condition may recur, but recurrences are usually milder than the initial bout.
Mucous Membrane Pemphigoid (Cicatricial Pemphigoid)
According to a guideline on mucous membrane pemphigoid (MMP) by the Task Force for Autoimmune Blistering Diseases of the European Academy of Dermatology and Venereology, mucous membrane pemphigoid is a group of autoimmune skin and mucous membrane blistering diseases, characterized by a chronic course and by predominant involvement of the mucous membrane.38 Mucous membrane pemphigoid should be considered a disease phenotype, with different conditions producing different autoantibodies targeting different autoantigens. Although histologically mucous membrane pemphigoid mimics bullous pemphigoid, this condition exhibits characteristic mucous membrane disease and very prominent scarring. Although rare, genital involvement is prominent and management can be challenging.
Clinical Presentation
Mucous membrane pemphigoid is much less common than bullous pemphigoid, but this phenotypic form of pemphigoid affects the mucous membrane of the genitalia, and it is often more difficult to diagnose. Many patients exhibit no intact blisters on extramucosal skin, and the prompt erosion of the mucous membrane blisters belie the underlying blistering nature of the condition. The age of onset of mucous membrane pemphigoid is between 60 and 80 years and is more common in women.38
Mucous membrane pemphigoid affects the genitalia in 28%-38% in two series.39,40 It affects keratinized skin only in about 20%-35% of cases.38 Because the diagnosis is difficult in the absence of intact blisters on hair-bearing skin, it may be delayed or sometimes missed. Mucous membrane pemphigoid usually starts with irritation, mild blisters, and erosions of the mouth, the eye, and the genitalia. Men report penile lesions, dysuria, and difficulty retracting the foreskin. Women report pain, pruritus, and dysuria. Morphologically, genital mucous membrane pemphigoid is characterized by painful erosions and scarring (Figs. 10-27 and 10-28). These mucous membrane erosions may progress rapidly to produce scarring with considerable morbidity. The initial, nonspecific, enlarging erosions produce scarring in more developed disease. Men may develop meatal stenosis and phimosis, and women may experience urethral stenosis, fusion of the labia, clitoral burial, and introitus stenosis.
Painful erosions in the mouth are usual (Fig. 10-29). Erythema and erosions of the gingiva may lead to scarring and retraction with resulting secondary dental disease. The esophagus can be involved as well. Nasal mucosa, larynx, and pharynx may also be affected, and stridor,
dysphonia, or dysphagia may result. The eye, despite feeling dry and gritty, may appear normal initially, but an ophthalmologic examination detects early abnormalities: reduced lacrimation or adhesions of the bulbar to the palpebral conjunctivae (Fig. 10-30). Later, more extensive scarring may cause severe adhesions, entropion, and corneal scarring with blindness. Nonmucous membrane lesions, when present, are very helpful by demonstrating the underlying blistering nature of the process. Lesions are small, straw-colored blisters that may scar.
dysphonia, or dysphagia may result. The eye, despite feeling dry and gritty, may appear normal initially, but an ophthalmologic examination detects early abnormalities: reduced lacrimation or adhesions of the bulbar to the palpebral conjunctivae (Fig. 10-30). Later, more extensive scarring may cause severe adhesions, entropion, and corneal scarring with blindness. Nonmucous membrane lesions, when present, are very helpful by demonstrating the underlying blistering nature of the process. Lesions are small, straw-colored blisters that may scar.
 Fig. 10-29. Erosive gingivitis indistinguishable from that of pemphigus vulgaris and erosive lichen planus is regularly seen in patients with mucous membrane pemphigoid. |
Diagnosis
The diagnosis is suspected by the constellation of ocular, oral, and genital irritation and erosions and a high index of suspicion and confirmed by the identification of antibasement membrane zone antibodies in nearby normal skin. There is linear deposition of IgG, IgA, and C3 at the BMZ. The diagnosis is often delayed, with resultant scarring. A biopsy for routine histology can be useful as well but is less sensitive and less specific. In the absence of a
blister, the edge of an erosion should be sampled. Histology shows a subepidermal blister, a mixed inflammatory infiltrate, and dermal scarring. Immunofluorescent studies of salt-split skin show the antibodies to be in the roof (epidermal) portion of the blister, although some are located in the floor (dermal) aspect of the blister.
blister, the edge of an erosion should be sampled. Histology shows a subepidermal blister, a mixed inflammatory infiltrate, and dermal scarring. Immunofluorescent studies of salt-split skin show the antibodies to be in the roof (epidermal) portion of the blister, although some are located in the floor (dermal) aspect of the blister.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree















