Fig. 60.1
Histograms of SpO2 and FiO2 show increased time within the target range of SpO2 and a striking reduction in time spent with high SpO2 during automated FiO2 adjustment compared to routine care. This is associated with exposure to lower FiO2 levels (Reproduced from Claure et al. (2009) with permission from Journal of Pediatrics)
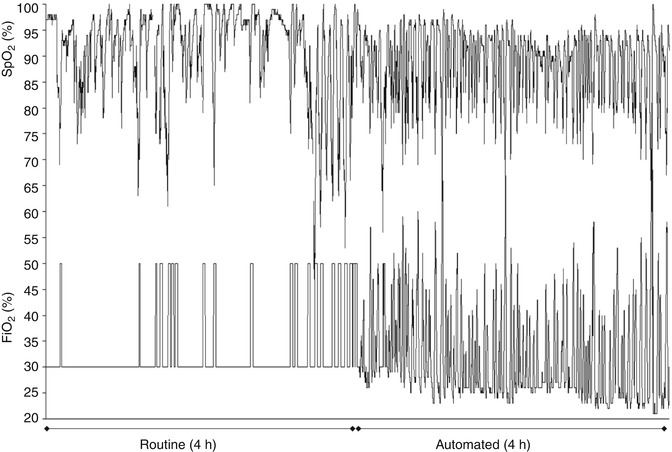
Fig. 60.2
Recordings of SpO2 and FiO2 during routine care show the caregiver response to episodes of hypoxemia. These are frequently followed by periods of hyperoxemia due to a persistently increased FiO2. In contrast, during the automated period there is a gradual reduction in the basal FiO2 which reduces hyperoxemia. However, this is accompanied by more frequent but relatively milder episodes of hypoxemia (Reproduced from Claure et al. (2009) with permission from Journal of Pediatrics)
The experiences with automated FiO2 adjustment also reveal potential respiratory effects of targeting different ranges of SpO2. A lower targeted range of SpO2 may reduce the infant’s oxygen reserves and lead to an increased susceptibility to hypoxemia spells from small fluctuations in ventilation that would otherwise cause minimal effects on arterial oxygen content.
60.3 Effects on Staff Workload
Data obtained in the studies evaluating automatic adjustment of FiO2 have also illustrated the effort required to maintain oxygenation within the intended range. As expected, the number of FiO2 adjustments by a dedicated caregiver was greater than those made by the routine caregivers and was also more effective in maintaining oxygenation within the intended range (Bhutani et al. 1992; Urschitz et al. 2004).
The effort spent in maintaining SpO2 within an intended range also depends on the frequency of fluctuations. As expected, those studies involving infants with frequent episodes of hypoxemia documented more frequent manual FiO2 adjustments. During routine care the caregiver must also be attentive to wean FiO2 to the basal level after a hypoxemia spell has resolved to avoid rebound hyperoxemia. This is commonly not done consistently and soon enough during routine care. This is illustrated in Fig. 60.2 where a routine caregiver was more attentive to intervene during episodes of hypoxemia than to prevent hyperoxemia. The gradual reduction in basal FiO2 during the automated period is accompanied by more frequent but milder episodes of hypoxemia.
60.4 Limitations
Continuous and accurate availability of the infant’s oxygenation is key in achieving an effective and safe automated adjustment of FiO2. The accuracy of pulse oximetry, the most commonly method used for automated FiO2 adjustment, can be affected by motion artifact. Poor accuracy can result in unnecessary exposure to supplemental oxygen levels during periods of falsely low SpO2 or prolonged hypoxemia if this goes undetected. These risks are present during routine care as well as during automated FiO2 adjustment. Other factors such as poor perfusion or inadequate probe placement and function can also reduce accuracy (Bucher et al. 1994). In general, conditions that impair SpO2 reliability and make its use questionable during routine care for an individual infant should also be considered as contraindications for its use for automated FiO2 adjustment.
The reliability of pulse oximetry in correctly identifying hypoxemia is important in automated FiO2 adjustment. Although most reports indicate reliable detection of hypoxemia by new pulse oximeters (Bohnhorst et al. 2000; Hay et al. 2002), some hypoxemia alarms during periods of infant activity are considered false due to the possibility of movement artifact. However, true hypoxemia spells can be associated with or result from the infant’s activity. These spells have been linked to increased activity and are more frequent when infants are awake (Bolivar et al. 1995; Esquer et al. 2007; Dimaguila et al. 1997; Lehtonen et al. 2002). This is important because artifactual hypoxemia spells during automated FiO2 adjustment can result in unnecessary oxygen exposure. Reassuringly, in infants with frequent hypoxemia spells, automatic FiO2 adjustment resulted only in minimal hyperoxemia overshoot (Claure et al. 2001, 2009). One would expect a high rate of hyperoxemia overshoot if most of the hypoxemia episodes were due to artifact.
Reduced attentiveness and poor observation of respiratory status may be an unwanted consequence of automated FiO2 adjustment. In some instances additional oxygen may only provide palliative support while not addressing the root cause of hypoxemia such as hypoventilation. To avoid this, automated systems must warn the caregiver when the automatically set FiO2 remains consistently elevated to maintain oxygenation in the desired range. Although this can be a potential drawback, it can be argued that the automatic increase in FiO2 is a necessary first step to maintain the infant properly oxygenated and reduce exposure to prolonged hypoxemia if the clinical intervention is delayed. Another important issue is the acceptance and adaptability of this form of automated support to the busy clinical environment of a newborn intensive care unit. This has been recently assessed under routine clinical conditions over 24-h periods without any adverse events (Claure et al. 2011).
The optimal range of oxygenation for preterm infants has not been determined. Exposure to high oxygenation levels has been shown to worsen respiratory and visual outcome with minimal or no beneficial effects. Observational studies where lower target ranges were used to avoid the effects of extremely high SpO2 suggested improved outcome (Tin et al. 2001; Chow et al. 2003; Wright et al. 2006; Vanderveen et al. 2006), but it is unknown how well those ranges were maintained and is possible the actual SpO2 may have exceeded those ranges. More recently, important evidence from a large trial indicated increased mortality with a lower SpO2 target range (SUPPORT Study Group of the Eunice Kennedy Shriver NICHD Neonatal Research Network et al. 2010). Because automatic FiO2 controllers will target a range set by the clinician, it is recommended that selection of the target range be done with caution and considering the possible physiologic effects of a more effective maintenance of a desired target range of SpO2 by the automatic FiO2 controller.
In summary, automated adjustment of FiO2 can improve maintenance of oxygenation within the intended range, reduce hyperoxemia and supplemental oxygen, and reduce staff workload. Larger clinical randomized trials are necessary to determine the effects of this automated form of support on survival and long-term respiratory, ophthalmic, and developmental outcome.
Essentials to Remember
Data indicate automatic FiO2 control can improve maintenance of oxygenation within the intended range while reducing hyperoxemia, supplemental oxygen, and workload compared to conventional manual adjustment of FiO2.
Automatic FiO2 controllers are designed to maintain the target range of oxygenation determined by the clinician.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


